INTRODUCTION
Stroke is a heterogeneous syndrome caused by the disruption of cerebral blood flow with subsequent tissue damage [1]. Epidemiologically, it is a serious neurological disease considered as the major cause of death and disability worldwide [2,3]. It can be classified into ischemic or hemorrhagic, with 85% of strokes being ischemic, an episode of neurological dysfunction caused by vascular stenosis or occlusion within a specific vascular territory [4].
Despite making up to only 2% of the total body weight, the brain consumes 20% of the body’s total energy and relies on a constant supply of glucose and oxygen to maintain its function and structural integrity [1]. Therefore, focal cerebral ischemia with severe hypoperfusion falling below the infarct threshold triggers a cascade of ischemic injury [5]. Therapeutic hypothermia (TH), which prevents irreversible neuronal necrosis and cerebral infarction, has strongly been investigated in animal studies and clinical trials with more effectiveness in postcardiac arrest and neonatal encephalopathy, primarily to prevent ischemia-reperfusion injury [6-8].
MECHANISMS OF ACTION OF THERAPEUTIC HYPOTHERMIA IN ACUTE ISCHEMIC INJURY
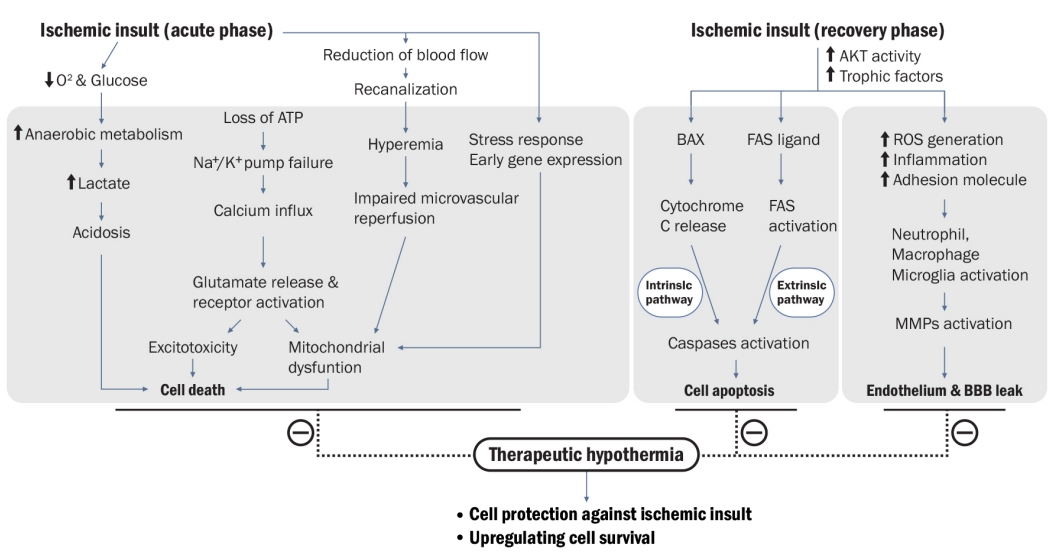
The brain injury mechanism after an ischemic stroke refers to the interaction of complex pathophysiological processes such as excitotoxicity, inflammatory pathways, oxidative damage, blood-brain barrier disruption, angiogenesis, and its restoration [4]. Current neuroprotection treatments for ischemic stroke injuries are not proven beneficial in the case of cerebral ischemia due to the complexity and disappointing results of various drug experiments in human clinical trials [9]. In contrast, TH is believed to inhibit or at least reduce the progression of this cascade at multiple levels (Fig. 1). Ischemic injury cascade starts with cerebral hypoxia, resulting in a loss of adenosine triphosphate (ATP) production and dysfunction of ATP-dependent Na+–K+ pumps in the cell membrane. The key cascades of this pathological process also commonly occur in various ischemic strokes. While the precise mechanism has not been fully understood, numerous hypotheses have suggested the neuroprotective effects of TH such as prevention of blood-brain barrier disruption; reduction of cerebral glucose metabolism and oxygen consumption; reduction of excitotoxic neurotransmitter accumulation, intracellular acidosis, intracellular calcium influx, and oxygen-free radical production; alteration of cold shock protein expression; reduction of brain edema; reduction of thrombosis risk; and reduction of the risk of epileptic activities [10]. In addition, TH can function as an antiedema therapy to prevent the increase in intracerebral pressure or impending cerebral herniation during an acute period of ischemic stroke [11]. In summary, potential mechanisms of TH can be described as “neuroglial protectors” for acute ischemic stroke.
COMPLICATIONS OF THERAPEUTIC HYPOTHERMIA
As mild hypothermia (34°C to 35.9°C) is relatively well tolerated, deep hypothermia (<32°C) appears to be related to more deleterious side effects directly caused by the intervention itself [12]. Physiological changes should be carefully considered and closely monitored on a daily basis in intensive care units (ICUs), and patients considering TH should be admitted to the ICU [13,14].
Side effects of TH can be categorized as cardiac, hematologic, immunologic, and metabolic complications [14,15]. Fig. 2 shows examples of complications during induction, maintenance, and rewarming of TH. Shivering in response to hypothermia can undermine the intentional goal of therapeutic cooling by generating heat that leads to increased core temperature and oxygen consumption. It is most prominent in the TH induction process; therefore, the use of sedatives and paralytics during this period should be carefully considered [16].
PREVIOUS STUDIES ON ACUTE ISCHEMIC STROKE
Several small phase II pilot trials have investigated the safety and feasibility of TH for acute ischemic stroke. These included the Cooling for Acute Ischemic Brain Damage (COOL-AID) trial [17]. This endovascular cooling trial demonstrated positive results of slower infarct growth in the TH group compared to that in the control group. As a result, the Intravascular Cooling in the treatment of stroke longer tissue plasminogen activator (tPA) window (ICTuS-L) was a phase I trial originally designed to establish the safety of endovascular cooling combined with tPA. Unfortunately, this trial was an eventual failure owing to a significantly increased proportion of pneumonia cases in the TH group [18].
For safety procedures and faster cooling than that in the previous ICTuS-L trial, the protocol of ICTuS 2 trial was modified in a prospective, multisite phase 2/3 pivotal, tissue plasminogen activator (tPA) trial combined with intravascular cooling catheter hypothermia in awake patients with moderate-to-severe middle cerebral artery (MCA) infarction with cold saline bolus and permissive hypothermia [19]. Unfortunately, this also did not show the usefulness of TH [20]. Recently, a phase III trial, an European randomized open-label clinical investigation with blinded outcome assessment (EuroHYP-1), provided no evidence that active cooling to a target of 34.0°C to 35.0°C for 12 to 24 hours initiated within 6 hours after the onset of ischemic stroke has an impact on the functional outcomes at 3 months [21]. This trial was discontinued after including 98 of the originally intended 1,500 patients because of slow recruitment and cessation of funding. This trial also was substantially underpowered to detect any clinically relevant benefit or harm [21]. Moreover, ICTuS 3 study poorly detected the clinical benefits owing to issues in patient enrollment during the study. These studies also raised questions about the feasibility of inducing hypothermia in relatively awake, spontaneously breathing stroke patients who tend to shiver vigorously and are prone to experience discomfort with hypothermia (unlike intubated and cardiac arrest patients).
Decompressive hemicraniectomy has been considered a powerful beneficial option to reduce the mortality of severely ill patients with a space-occupying MCA infarction; however, a considerable number of patients are still suffering from serious disability or even face death. Therefore, TH can be clinically beneficial in those with severe stroke treated with hemicraniectomy because of its strong antiedema therapy during the acute phase of ischemic stroke. Nonetheless, several clinical data demonstrated that hypothermia treatment had no additional benefits on the functional outcome as compared with hemicraniectomy alone [11,22,23]. Therefore, we should include specific candidates who would be more feasible targets for detecting the clinical efficacy and safety of TH in future trials.
COMPARATIVE LESSONS ON THERAPEUTIC HYPOTHERMIA: SUCCESSFUL GLOBAL ISCHEMIA VS. FAILED FOCAL ISCHEMIA
Since 2002, when the trial “Hypothermia after Cardiac Arrest (HACA)” study group demonstrated the clinical benefits of TH in improving neurological and mortality outcomes in postcardiac arrest patients with shockable rhythms, many studies have shown the positive effects of hypothermia on the neuronal protection in global brain ischemia [6,7].
However, the evidence of TH for focal cerebral ischemia in stroke remains inconclusive. Preclinical studies support that TH is much more beneficial and consistent in temporary MCA occlusion than in permanent MCA models [24]. In addition, several studies reported that despite the association of TH with increased risk of pneumonia, longer duration of ICU stay, and prolonged mechanical ventilation dependency, these factors did not affect the neurological outcome and ICU survival [25]. Compared to the conventional TH guideline on postcardiac arrest syndrome, some data showed a beneficial tendency in case of longer duration of cooling and rewarming in cardiac arrest [26], ischemic stroke [27], and traumatic brain injury [28]. Generally, clinical deterioration after an ischemic stroke attributable to HT and cerebral edema usually occurs between 2 and 5 days after stroke [29]. The time course of edema after stroke and extrapolation from traumatic brain injury hypothermia studies suggesting a prolonged course of TH with slow and controlled rewarming may be important for the success of TH protocols in patients with stroke. Therefore, animal and clinical studies suggest that TH with a long-durational and specific protocol might be more effective in patients with severe stroke who underwent successful recanalization by preventing ischemia-reperfusion injury in endotracheal intubation obligation such as cardiac arrest or perinatal postanoxic status.
LESSONS FROM PREVIOUS IN VIVO EXPERIMENTS
Although some trials showed positive efficacy of longer TH duration from different clinical situations, its optimal duration remains unclear. Considering that neurovascular damage after an acute ischemic attack occurs over hours to days, the longer the duration of hypothermia is, the more significantly improved the outcomes can be. In support of this hypothesis, some experiments have already demonstrated better outcomes with longer duration of cooling [10]. Interestingly, van der Worp et al. [30] concretely reported an inverse relationship between the duration of TH and infarct volume in a systemic review and meta-analysis of animal models. As compared to shorter (3 hours) periods of hypothermia in Sprague-Dawley rats with transient middle cerebral artery occlusion (tMCAO), infarct size reduction was greater and better outcomes were observed in patients treated in longer hours (21 hours) [31]. This can lead to an expansion of time window and number of patients for the treatment of endovascular recanalization in patients who had emergent large vessel occlusion (ELVO) stroke. Although no guidelines have recommended the exact therapeutic timeframe for TH, time-sensitive characteristics of hypothermia induction cannot be easily disputed due to solid results of previous experiments [32]. In summary, available experimental data showed that TH could be (1) more successful when applied quickly; (2) when applied with sufficient duration; and (3) when applied to the ischemia-reperfusion model.
TARGETED TEMPERATURE MANAGEMENT DURING OR AFTER AN ENDOVASCULAR RECANALIZATION
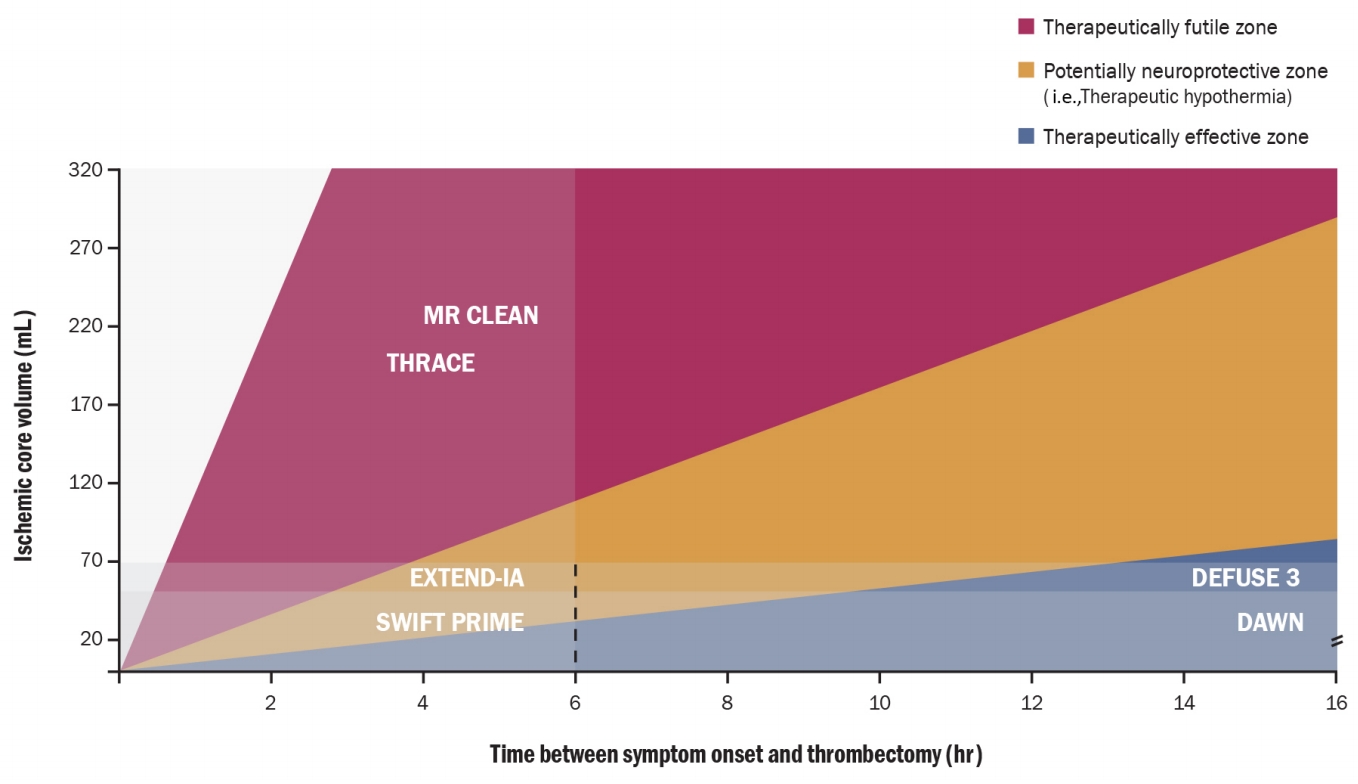
Endovascular treatment (EVT) such as mechanical clot retrieval has become a proven therapeutic strategy for acute ischemic stroke with an ELVO recently [33]. Many of these patients are at high risk of brain injury owing to their large stroke volume and of reperfusion injury even if recanalization was successful. Among the neuroprotective strategies, TH has been attempted to evaluate the outcome benefits in experiments and clinical trials [4,9]. In a recent postreperfusion TH study, several patients (approximately 45%) had favorable outcomes based on the modified Rankin Scale 0, 1, 2 at 3 months despite low baseline alberta stroke program early CT score (ASPECTS) and large lesion volume (>80 mL) [34]. Recent EVT trials showed that EVT was useful for patients with a large core volume, and reperfusion rate was a predictor of good outcomes [35-37]. However, several studies in patients with malignant MCA trait demonstrated that low ASPECTS on computed tomography (initially large parenchymal lesion) was a predictor of poor outcomes despite successful recanalization. Such contradictory results demonstrate that reperfusion itself does not always guarantee a good functional outcome, especially in patients with “malignant MCA infarction trait” [33,38]. Neuroprotection after recanalization is evident in the EVT era to improve the clinical outcomes of these contradictory groups. Failure of previous neuroprotective drugs can be overcome by achieving endovascular recanalization, a similar mode of ischemic-reperfusion model in preclinical experiments. In this context, immediate postreperfusion cooling can be a promising option to minimize reperfusion-related complications. The rate of infarct growth has also been known to vary depending on an individual’s diversity, such as the collateral blood flow in patients with acute ischemic stroke. Recent success of the late window EVT trials may be because of the selection of “slow progressors” in the infarct growth process [35] In the future, neuroprotective modalities, including TH and other agents for reperfusion injury, may be promising for patients not classified as either fast and slow progressors (Fig. 3). A recent study has shown that neurocritical care treatments including TH are feasible even in patients with a malignant infarct core [39]. Therefore, the strategy reducing reperfusion injury using TH will be another option for vulnerable patients suffering from acute ischemic stroke in the EVT era. Although TH protocols remain debatable [40], it can be com bined with existing therapies to improve outcomes of patients with acute ischemic stroke through the technological development of EVT.