INTRODUCTION
Accurate neurological prognostication after brain injury can be exceedingly challenging. In the setting of numerous confounders, such as multi-drug intoxication or overdose, predicting neurological outcomes becomes even more difficult. We describe a case of an intentional overdose of multiple medications, specifically involving bupropion, complicated by cardiopulmonary arrest, renal failure, and the loss of brainstem reflexes followed by a full neurological recovery.
CASE REPORT
A 31-year-old man with a history of longstanding depression and anxiety was brought to the emergency department with acute encephalopathy after a presumed suicide attempt. Emergency medical services found him disoriented and agitated at his residence with several empty prescription containers of mirtazapine, oxcarbazepine, fluoxetine, sustained-release bupropion (bupropion SR), gabapentin, and hydroxyzine; all bottles were confirmed by his significant other to be nearly full the night prior. Outpatient pharmacy records corroborated a recently dispensed 30 day supply of bupropion SR 200 mg to be taken every 12 hours; this suggests an approximate ingestion dose of 12 g.
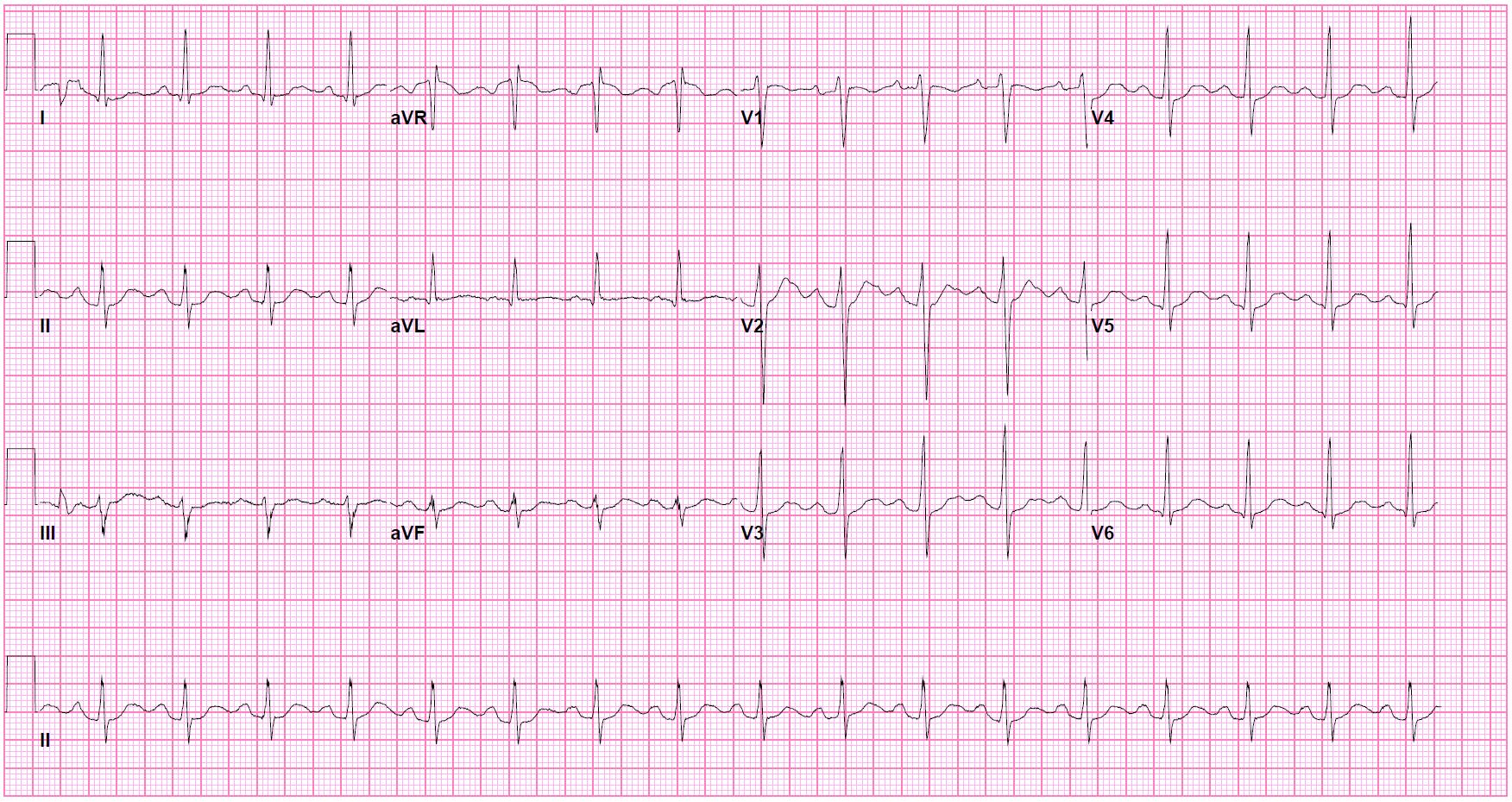
Upon presentation to the hospital, vital signs were normal aside for mild tachycardia at 102 beats/min. General physical examination was notable for dry mucous membranes and decreased bowel sounds. Neurologically, he was lethargic and only arousable to pain but was able to state his name and was protecting his airway. Pupils were 7 mm, symmetric, and briskly reactive bilaterally with the remainder of the exam only significant for diffuse hyperreflexia and symmetric localization to noxious stimulation in all extremities; no myoclonus or abnormal movements were observed. Initial laboratory evaluation revealed a moderate leukocytosis (27×109/L), an anion-gap metabolic acidosis (anion gap 19 mmol/L, CO2 19 mmol/L), and an elevated lactate (6.4 mmol/L). The patient tested positive for amphetamines, benzodiazepines (which had been given pre-hospital), and cannabinoids. Other diagnostics, including an electrocardiogram (EKG) and computed tomography (CT) of the head and cervical spine, were unremarkable (Fig. 1). Activated charcoal and bowel irrigation were not utilized because of the unknown time of ingestion.
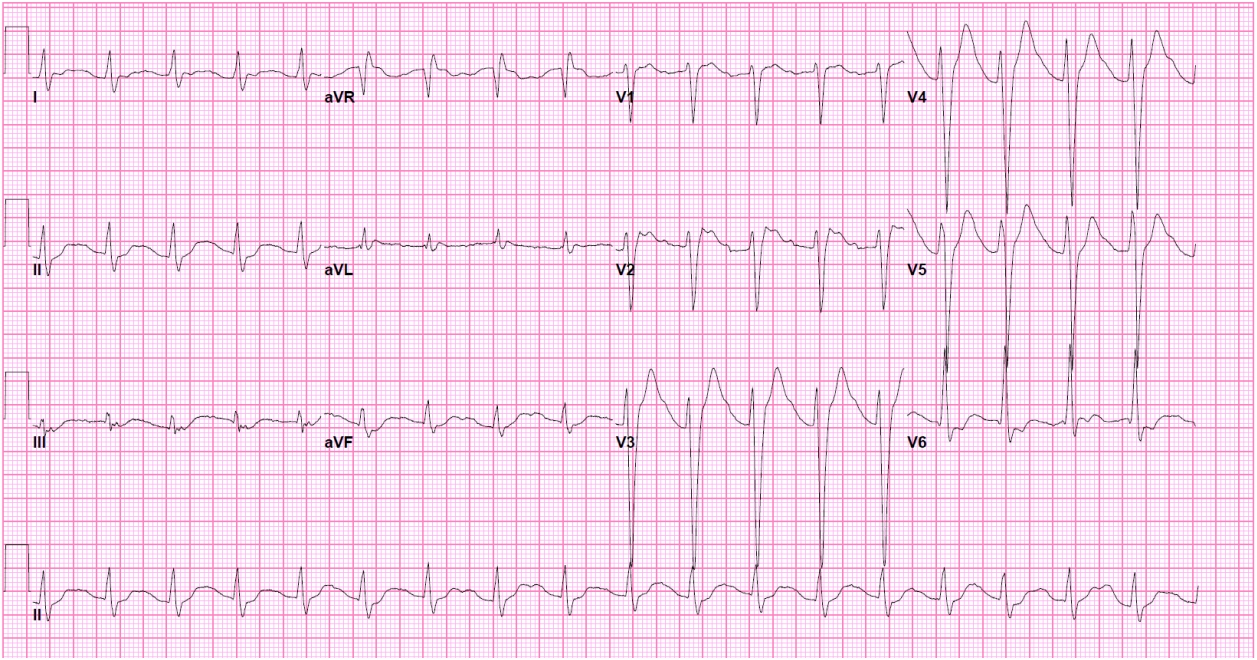
Several hours after admission the patient suffered two cardiac arrests characterized by pulseless electrical activity. Return of spontaneous circulation (ROSC) was achieved after 14 and 16 minutes, respectively, of high-quality and timely cardiopulmonary resuscitation. Resuscitation medications included multiple doses of epinephrine, sodium bicarbonate, and crystalloid solution; no sedatives or paralytics were given during the intubation. An EKG showed a new first degree atrioventricular block with slight QRS prolongation at 144 ms (Fig. 2). Serial neurological examinations immediately after the arrests and over the course of several hours consistently demonstrated absent corneal reflexes bilaterally (no blink with saline or tactile stimulation in either eye), no cough reflex with suction catheter stimulation, no oculocephalic reflex with horizontal and vertical head rotation, and no appreciable motor response to pain in any extremity; each pupil was dilated at 7 mm and unreactive to direct and indirect light. The decision was then made to administer lipid emulsion therapy and begin targeted temperature management (TTM) with a goal of 33°C Celsius. Despite aggressive measures, he deteriorated further and suffered widespread multi-organ damage evidenced by shock liver and acute non-oliguric renal failure necessitating continuous renal replacement therapy. Prior to the cooling process, a continuous electroencephalogram (cEEG) revealed a burst suppression pattern which remained unchanged over a 2-day recording period. A repeat CT of the brain acquired 48 hours after ROSC was consistent with diffuse cerebral edema and exhibited interval development of sulcal and cisternal effacement and subtle blurring of the grey-white matter interface when compared to his initial scan (Fig. 3). Bedside evaluation at the initiation of TTM had been notable for persistent absence of pupillary, corneal, oculocephalic, and cough reflexes and the lack of any motor reaction to noxious stimulation as previously described. Two days after rewarming was completed and all sedatives had been discontinued—fentanyl and midazolam infusions were utilized for TTM—no brainstem reflexes (pupillary, corneal, oculocephalic, and cough) and no motor response in any extremity could be elicited on examination. Immediate neurological prognostication and formal brain death testing were not yet pursued, however, due to the recent completion of the targeted temperature protocol and the suspected confounder of ingestion.
On hospital day (HD) 6, the patient regained bilateral corneal and pupillary reflexes. By HD 8, he was conversing and following commands without difficulty. Throughout the remainder of the admission, his overall function continued to rapidly improve. He was eventually discharged on HD 18 with a completely normal neurological examination and no evidence of cognitive impairment on formal neuropsychological testing.
DISCUSSION
Bupropion is a selective dopamine and norepinephrine reuptake inhibitor utilized in the treatment of multiple conditions including clinical depression, attention deficit/hyperactivity disorder, and seasonal affective disorder. It also has adjunct benefits for neuropathic pain, smoking cessation, and weight loss [1]. The most commonly seen side effects are dry mouth, nausea/vomiting, agitation, gastrointestinal discomfort, and dizziness. Serious complications include cardiac abnormalities such as tachycardia and arrhythmias (QRS widening, QT prolongation); cases of cardiogenic shock have also been reported [2]. Significant central nervous system effects consist of hallucinations, agitation, seizures, tremors, stupor, and coma. Bupropion demonstrates linear pharmacokinetics and is hepatically processed into several metabolites—hydroxybupropion, erythrohydrobupropion, and threohydrobupropion. These metabolites, all of which are considered active and whose concentrations rise above their parent drug level, have an elimination half-life of >20 hours and are eventually renally excreted. Peak plasma concentrations for the SR and XL formulations are reached after 3 and 5 hours, respectively [1,3].
Management of bupropion overdose is primarily supportive and is characterized by standard emergency protocols for airway securement, oxygenation/ventilation, and hemodynamic stabilization; no known antidote exists. Seizures should be treated accordingly, and cEEG monitoring is advised for the first 48 hours since delayed onset of clinical seizures have been observed beyond 14 hours after consumption [4]. Interventions such as gastric irrigation or activated charcoal are generally recommended if ingestion occurs within an hour of presentation. Lipid emulsion therapy in bupropion overdose is an alternative but evidence is controversial and scarce though favorable outcomes have been observed; while not considered a first-line treatment, it may be beneficial in patients with delayed or prolonged deterioration and in those who suffer a cardiac arrest [2].
We acknowledge that the fixed, bilateral mydriasis and poor clinical exam could have been due to the cardiac arrests; both the intoxication and the cardiopulmonary events are undoubtedly contributors to the clinical picture. However, the rapid and complete clinical improvement may suggest a predominantly drug effect. We propose bupropion should be considered a clinically important confounder with unique and dramatic effects on the neurological examination. There have been several descriptions of comatose patients with fixed mydriasis and a burst suppression cEEG secondary to bupropion overdose but without an accompanying cardiopulmonary arrest [5-7]. This supports the notion of bupropion playing a role in a clinically comatose patient apart from any concurrent anoxic brain injury. Similarly, to what degree hypoxia versus possible delayed effects of bupropion were each responsible for the development of cerebral edema in our patient is also unclear. Normal CT findings have been documented with larger ingestion quantities, though imaging was typically obtained solely on admission in these cases; another report recorded diffuse brain edema only after a prolonged arrest [6]. Our case adds to the growing body of literature which suggests bupropion, when consumed in excessively large amounts, might be associated with a disproportionately poor neurological exam. TTM and coexisting renal failure were suspected to also play a role. Furthermore, our patient uniquely illustrates that a full neurological recovery can still be expected despite an ominous clinical course involving multiple cardiopulmonary arrests, organ failure, and the presence of a neurological insult on head imaging. The precise mechanism by which bupropion intoxication can have such a profound impact on the clinical exam is unknown but its modulatory effects on both noradrenaline and dopamine throughout the brainstem have been implicated. Noradrenergic alterations in the locus coeruleus, for example, influence pupillary size even in the absence of luminescent stimulation [8].
Although the patient ingested numerous medications in excess to bupropion, these alternatives were unlikely to be the primary culprit of his poor neurological status given their well-documented toxicology profiles. Mirtazapine has been shown to be largely benign even with overdose of extreme quantities. When consumed along with other substances, the co-ingested drugs are typically deemed the source of severe clinical symptoms [9]. Serotonin syndrome from fluoxetine, a commonly used selective serotonin reuptake inhibitor, is well described along with seizures and cardiac conduction delays [10]. Though coma and poor outcomes have been described with oxcarbazepine, they are infrequent and are typically the result of uncontrolled seizures or cardiac conduction irregularities [11]. Lastly, mortality secondary to acute intoxications of gabapentin and hydroxyzine have been reported but are extremely rare; no reports of clinical examinations consistent with brain death have been identified with these medications [12,13].
Our case also reiterates the value and relevance of pharmacodynamics and pharmacokinetics in the assessment of brain injury related to medication overdose. Slower absorption of delayed-release or sustained-release formulations could rationalize the use for employing bowel irrigation or activated charcoal, both of which are usually avoided in late presentations or when time of ingestion is unknown. Likewise, concurrent anticholinergic toxidromes (e.g., hydroxyzine is known to have some affinity for muscarinic acetylcholine receptors), can hinder gastrointestinal motility and absorption and further worsen drug metabolism. Utilization of the aforementioned interventions beyond the customary 1-hour time period in situations where gastric absorption might be impaired (e.g., hypothermia) therefore warrants special consideration.
Finally, the current vignette also accentuates the importance of clinical equipoise in neurological prognostication and brain death evaluations after acute intoxications. Clinicians must not only be aware of potentially significant laboratory derangements related to renal or hepatic injury but must also appreciate how damage to these organs, along with hypothermia, alter systemic metabolism and clearance. Caution must be exercised when numerous substances are involved, and providers should recognize how interventions such as renal replacement therapy may or may not facilitate toxin or medication excretion. Assuming normal hepatic and renal function, formal brain death testing is routinely delayed until at least five half-lives have passed after administration of a potential clinical confounder such as a paralytic or sedative; this time period should be extended even further in the presence of liver and/or kidney damage [14]. In instances where massive overdoses of psychotropic medications are implicated, many of which possess half-lives on the order of days, erring towards a more cautious approach is justified.
Bupropion overdose is associated with a clinical examination demonstrating the absence of brainstem reflexes and the lack of a meaningful motor response, the combination of which can be suggestive of brain death in the proper clinical setting. Despite these worrisome findings, however, a full neurological recovery can still be expected. Special consideration should be given towards the delayed use of gastric or whole bowel irrigation, activated charcoal, and lipid emulsion therapy in specific scenarios. Neurologists and intensivists should be aware of this brain death mimic and be judicious in their efforts to offer prognostication, especially in the setting of various confounders such as cardiac arrest, acute renal or hepatic failure, and TTM.