Refractory brainstem encephalitis mimicking progressive cerebral infarction: infliximab and methotrexate as a salvage immunotherapy
Article information
Abstract
Background
Brainstem encephalitis is a rare, severe, and potentially life-threatening inflammation of the central nervous system, exhibiting various treatment responses and outcomes owing to multiple etiologies.
Case Report
We describe the favorable outcome of salvage immunotherapy using a combination of infliximab and methotrexate in a 62-year-old woman with refractory brainstem encephalitis. The patient was initially presumed to be at a subacute stage of medullary infarction but showed progressively worsening conditions involving cervical myelopathy, despite having completed the schedule of subsequent immunotherapy with intravenous methylprednisolone, immunoglobulin, and rituximab. After completion of four sessions of weekly rituximab injection, she was treated with 5 mg/kg of infliximab, scheduled at 0, 2, and 6 weeks, along with methotrexate (weekly 12.5 mg). After completion of infliximab injection and maintenance with methotrexate treatment, she showed an improving course of quadriplegia.
Conclusion
This case report provides evidence for the potential efficacy of infliximab with methotrexate in cases of refractory brainstem encephalitis.
INTRODUCTION
Brainstem encephalitis is a rare, severe, and potentially life-threatening inflammation of the central nervous system that exhibits various treatment responses and outcomes owing to multiple etiologies [1]. However, owing to the risk of surgical biopsy of vulnerable structures such as the brainstem, the etiological evaluation of neuroinflammatory and immune-mediated brainstem lesions has been limited [2]. There is a need for detailed clinical assessment and investigations using magnetic resonance imaging (MRI) for detection of lesion location, distribution, and morphology, and cerebrospinal fluid (CSF) and serum studies for detection of infectious sources, autoimmune and paraneoplastic antibodies, and others [3].
Besides the difficulty in differential diagnosis of various inflammatory diseases in the brainstem, determining the ideal candidates for therapeutic management of brainstem encephalitis is difficult and a serious concern, especially in worsening cases where the causative pathogen has not been detected. Thus, conventional immunotherapy using intravenous steroids and immunoglobulin has been considered for empiric immune therapy because of the lack of controlled trials for the treatment of related disorders [4]. Furthermore, recent studies have suggested that autoimmunity is an emerging and common etiology of cryptogenic encephalitis [5]. Based on this estimation, early intensive immunotherapies are recommended in cases without evidence of infectious causes and in those where specific antibodies are not detected [6].
We describe a 62-year-old woman who presented with a catastrophic and rapid deterioration resulting in quadriplegia, which was initially suspected due to refractory cerebral infarction to the intensive stroke management. This was further aggravated despite subsequent immunotherapy with intravenous methylprednisolone, immunoglobulin, and rituximab under the assumption of cryptogenic brainstem encephalitis with cervical spinal cord involvement, until the completion of infliximab treatment as salvage immunotherapy.
CASE REPORT
A 62-year-old woman without any previous history of atherosclerotic risk factors or vascular and autoimmune disease was transferred to our center presenting with sudden onset of hiccups and paresthesia on the right face and limbs 5 days prior. Baseline laboratory tests revealed no definite evidence of systemic inflammation or infection. Brain MRI showed an increased diffusion-weighted image (DWI) sequence at the right medial medulla with a combination of an increased T2-weighted and T2-weighted fluid-attenuated inversion recovery (FLAIR) sequence signal intensity and normal apparent diffusion coefficient (ADC) sequence (Fig. 1). Under the assumption of a subacute stage of infarction related to small vessel occlusion due to nonremarkable angiography, she was administered oral aspirin 100 mg, clopidogrel 75 mg, and atorvastatin 40 mg, and intravenous fluid maintenance.

Magnetic resonance imaging (MRI) findings at admission. Baseline brain MRI revealing increased signals on the diffusion-weighted imaging sequence on the right anteromedial medulla (A) with a subtle signal change on apparent diffusion coefficient sequence (B) and increased signal intensity on T2-weighted (C) and T2-weighted fluid-attenuated inversion recovery (D) sequences.
However, by day 5 of hospitalization, the episode of paresthesia on the right face and limbs became more frequent and worse, accompanied by fluctuating motor weakness in the left limbs and hoarseness. Therapeutic-induced hypertension and triple anti-platelet agents including Cilostazol 200 mg were additionally administered, otherwise these problems were still worsening. Transfemoral cerebral angiography revealed no definite evidence of vascular abnormalities such as dissection, arteriovenous malformation, or fistula in the vertebrobasilar and spinal artery; intra-arterial and intravenous tirofiban treatment was then provided.
On day 13 of hospitalization, neurological deficits worsened further, and follow-up MRI of the brain and spine revealed a persistent signal change on the DWI sequence with an aggravated perilesional T2-weighted and T2-weighted FLAIR signal intensity and enhancement in the medulla oblongata with expansion to the C1 level of the spinal cord, without evidence of intraventricular and periventricular ependymal lesions (Fig. 2). At this stage, we considered brainstem encephalitis based on the aggravated MRI findings compatible with vasogenic edema and performed a workup for etiological diagnosis before immunotherapy. She denied any previous history of autoimmune diseases such as recurrent oral or genital ulcers, skin problems, ophthalmoplegia, ophthalmodynia or visual acuity problem, and limb paresthesia, and had normal findings on fundoscopic and visual field tests on ophthalmologic evaluation. Serum anti-aquaporin-4 antibodies were absent; we did not perform further evaluation of the myelin oligodendrocyte glycoprotein (MOG) and HLA-B51 test focusing on the possible diagnosis of Bechet disease or anti-MOG associated inflammatory demyelinating diseases.
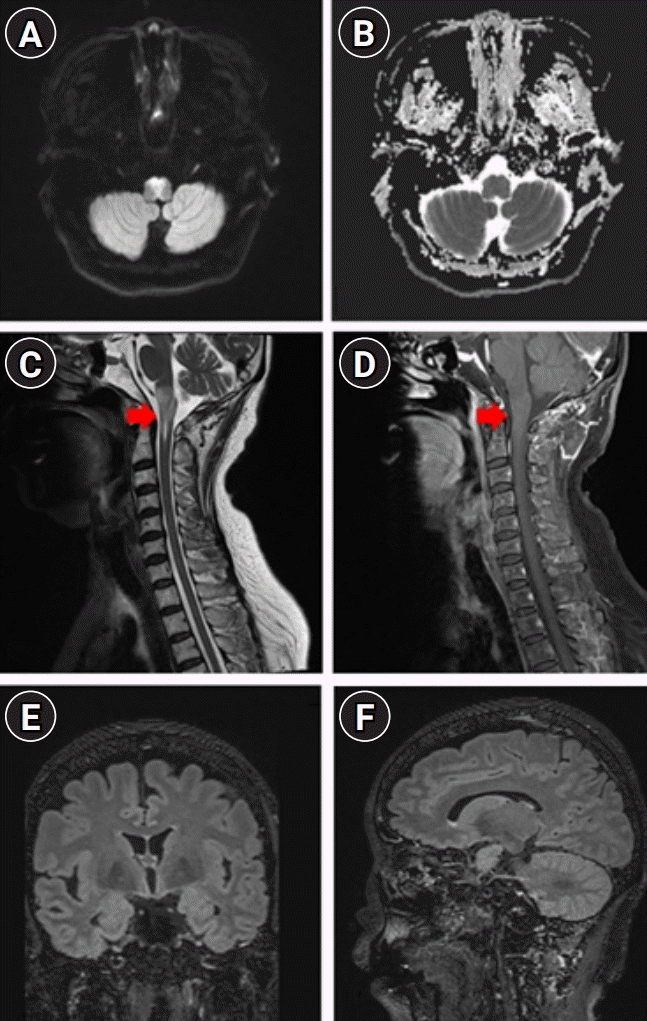
Magnetic resonance imaging (MRI) findings at 2 weeks of hospitalization during management for infarction. Follow-up brain spine MRI revealing further increased signals on the diffusion-weighted imaging sequence on the bilateral anteromedial medulla (A) with a subtle signal change on apparent diffusion coefficient sequence (B) and increased signal intensity on T2 sequence within C1 level of the medullar oblongata and spinal cord with increased enhancement (red arrows; C, D, respectively) during the therapeutic-induced hypertension and anti-thrombotic treatment, including triple anti-platelet agents and tirofiban infusion without evidence of intraventricular and periventricular ependymal lesions (E, F).
CSF analysis was unremarkable, except for lymphocytic pleocytosis: cells 19/μL, protein 41.0 mg/dL, glucose 64 mg/dL, and lactate dehydrogenase below 50 IU/L. Oligoclonal bands were absent with mild elevation of albumin (31 mg/dL) and immunoglobulin G (5.45 mg/dL) in CSF. CSF stain and culture, and polymerase chain reaction test results for the detection of a wide range of bacteria, tuberculosis, fungi, and viruses, and cytospin tests for the detection of malignant cells were negative. Test results for the detection of serum influenza virus A/B antigen, antibodies (e.g., herpes simplex, varicella zoster, Hantaan, Leptospira, tsutsugamushi, Toxocara, and mycoplasma), blood culture, autoimmune tests (e.g., anti-nuclear, anti-double-stranded DNA, anti-thyroid peroxidase, anti-thyroglobulin, and anti-neutrophilic cytoplasmic antibodies, rheumatoid factor, and lupus anticoagulant), and immunofixation were negative. The serum angiotensin-converting enzyme levels were within the normal range. Tests for the detection of anti-ganglioside antibodies, including GQ1b, were negative in the serum. Computed tomography of the chest and abdomen and whole-body positron emission tomography revealed no definite evidence of malignancy. Test results for the detection of autoimmune encephalitis antibodies (NMDAR, AMPA1, AMPA2, LGI1, CASPR2, and GABA-B) and paraneoplastic antibodies (Hu, Yo, Ri, Ma2, CV2/CRMP5, amphiphysin, recoverin, SOX1, and titin) were negative.
Under the assumption of cryptogenic brainstem encephalitis, she received sequential treatment with 5 days each of 1,000 mg of intravenous methylprednisolone and 0.4 g/kg of intravenous immunoglobulin. However, she showed deteriorated quadriparesis with grade 2 on the Medical Research Council (MRC) scale accompanied with painful spasms and spasticity. She was then retreated with intravenous methylprednisolone for 5 days, followed by 375 mg/m2 of rituximab treatment. However, despite completion of 4 weeks of weekly rituximab injection, neurological deficits worsened further to quadriplegia with grade 0 on the MRC scale accompanied with frequent spasms at intervals of several minutes resistant to multiple anti-epileptic drugs. Repeated examination of serum and CSF including all of the above-mentioned profiles revealed no significant difference compared to baseline findings, and follow-up brain MRI showed further expansion of diffusion-restricted lesions with increased perilesional signal intensity on T2-weighted and T2-weighted FLAIR sequences (Fig. 3). At this stage, we decided to treat her with 5 mg/kg of infliximab scheduled at 0, 2, and 6 weeks according to the manual for intractable Crohn disease with a combination of methotrexate (weekly 12.5 mg) for modulation of cell-mediated immunity to complement the prior immunotherapies mainly focusing on humoral immunity. After completion of infliximab injection with maintenance with methotrexate treatment, she showed an improving course of quadriparesis with grade 2 on the MRC scale and decreased frequency of spasms. Repeated CSF measurements showed an improving course of pleocytosis (from 19, 17, 11 to 2/µL) with persistently elevated protein (from 41.3, 46.9, 59.6 to 61.3 mg/dL) at baseline, 1 month (after completion of intravenous immunoglobulin and methylprednisolone), 2 months (after completion of rituximab treatment), and 3 months (after completion of the 2nd injection of infliximab). After 7 months of onset (3 months after completion of infliximab treatment), she was able to sit alone with further improvement of quadriparesis with grade 4 on the MRC scale. Follow-up brain MRI revealed a decreased extent of abnormal T2-weighted and T2-weighed FLAIR high signal intensity in the bilateral anteromedial medulla and cervical spinal cord with normalized DWI and ADC sequences (Fig. 4).

Magnetic resonance imaging (MRI) findings at 2 months of hospitalization after completion of conventional immunotherapy. Follow-up brain MRI revealing expansion of diffusion-restricted lesions on the diffusion-weighted image sequence to both the anteromedial medulla (A) with a subtle signal change on apparent diffusion coefficient sequence (B) and increased perilesional signal intensity on T2-weighted (C) and T2-weighted fluid-attenuated inversion recovery (D) sequences, after completion of immunotherapy including intravenous methylprednisolone and immunoglobulin following completion of four sessions of weekly rituximab treatment.

Magnetic resonance imaging (MRI) findings at 10 months after symptom onset. Follow-up brain MRI revealing a normalized diffusion-weighted image (A) and apparent diffusion coefficient (B) sequence on both the anteromedial medullar and cervical spinal cord with decreased perilesional signal intensity on T2-weighted (C) and T2-weighted fluid-attenuated inversion recovery (D) sequences, 6 months after completion of infliximab treatment.
DISCUSSION
We describe the favorable outcome of salvage immunotherapy using infliximab in combination with methotrexate in a 62-year-old female with refractory brainstem encephalitis, who was initially presumed to be at a subacute stage of medullary infarction with progressively worsening conditions despite having completed the schedule of subsequent immunotherapy with intravenous methylprednisolone, immunoglobulin, and rituximab.
Until now, the etiologies of brainstem encephalitis have been poorly studied; although several entities, such as Listeria rhombencephalitis, Bickerstaff’s brainstem encephalitis, paraneoplastic encephalitis, and neurosarcoidosis have been suggested as possible causes [2,7], the spectrum of causes and outcomes of brainstem encephalitis are unclear [1,8]. In our case, we failed to reveal the possible etiologies despite the broad investigation of CSF and serum with repeated measurements, due to lack of results from the high-risk needle biopsy of the upper cervical medulla oblongata. Furthermore, we also failed to achieve a therapeutic response with rituximab as adjunctive immunotherapy in addition to the conventional immunotherapy based on the current concept for the management of broad-spectrum encephalitis; several recent systematic reviews have recognized that early intensive immunotherapy is associated with better outcomes in autoimmune encephalitis [5]. Our case also showed a unique course of gradual aggravation of neurological deficit to complete quadriplegia until 14 weeks after symptom onset, and then achieved a gradual improvement of neurological deficit for 14 weeks after completion of intravenous immunotherapy with a maintenance of oral methotrexate (Fig. 5).
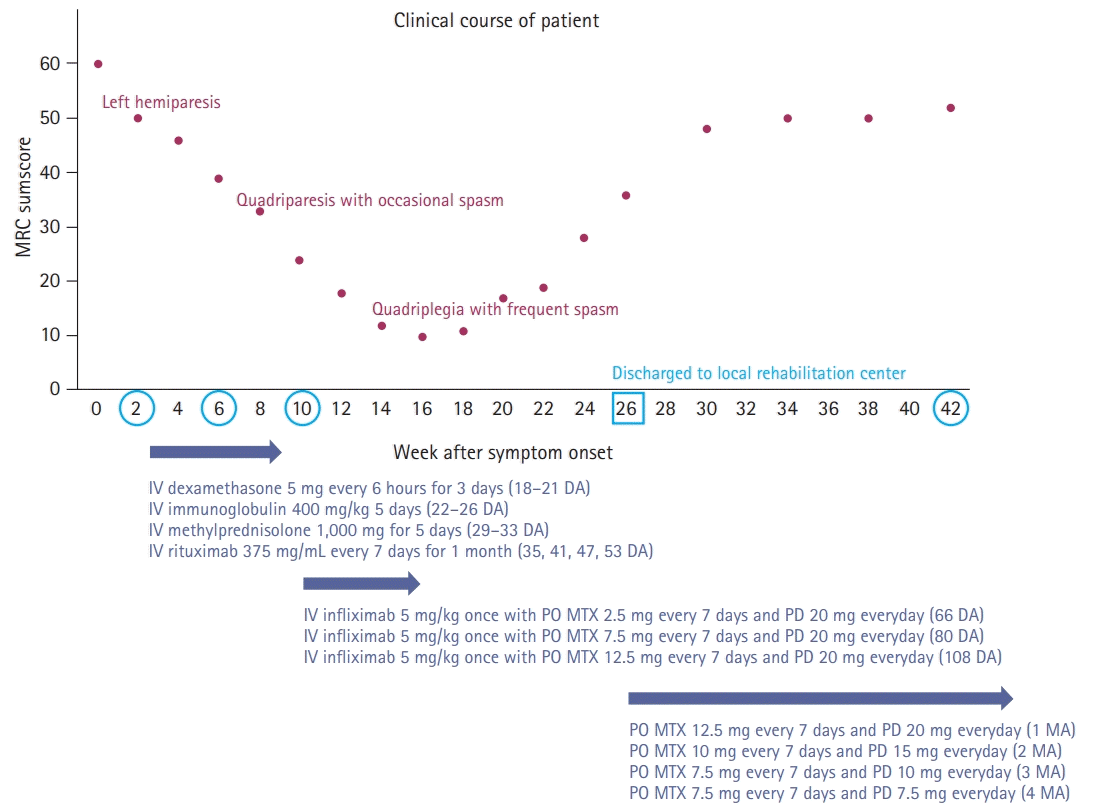
Clinical course of patient. The patient’s neurological deficit (measured by Medical Research Council [MRC] sumscore) and magnetic resonance imaging findings (blue circle) worsened until 18 weeks and gradually improved after completion of the scheduled escalation of immunotherapy until 16 weeks and maintenance of PO MTX and PD, following gradual tapering after discharge (blue square) at 26 weeks after symptom onset. MRC sumscore evaluates global muscle strength including manual strength of six muscle groups (shoulder abduction, elbow flexion, wrist extension, hip flexion, knee extension, and ankle dorsiflexion) on both sides using the MRC scale. Summation of scores gives MRC sumscore, ranging from 0 to 60 (0–36, quadriplegia to severe quadriparesis; 36–48, mild quadriparesis; 48–60 [max], normal strength). IV, intravenous; DA, days after onset; PO, per oral; MTX, methotrexate; PD, prednisolone; MA, months after discharge.
Thus, considering refractory brainstem encephalitis, including intractable myelopathy, we recognized that neurosarcoidosis was the most common etiology of cryptogenic cord problems based on biopsy results [9,10], and management with global immunosuppressant agents in combination with immunomodulatory monoclonal antibodies, such as infliximab, was considered to demonstrate effective management [11,12]. Infliximab can be considered as salvage immunotherapy in addition to rituximab, considering the different mechanisms of immunomodulation. It is a chimeric monoclonal antibody against tumor necrosis factor-α (TNF-α) and has emerged as a treatment option in refractory and steroid-dependent patients with neurosarcoidosis [12] as TNF-α is a cytokine critical for granuloma formation and maintenance in human sarcoidosis granulomas [11]. In contrast, rituximab is a monoclonal antibody against CD20-positive B cells and induces B-cell depletion, and consequently leads to suppression of autoimmune neurological disorders via modulation of immunity [13], with relative safety in terms of infectious adverse effects. Therefore, despite the lack of biopsy results, we attempted to treat the patient using infliximab as an adjunctive salvage immunotherapy and achieved gradual improvement in clinical features, as well as in findings of radiographic (mainly perilesional vasogenic edema) and CSF (pleocytosis) examinations. In addition, methotrexate has been well-documented for its efficacy in the treatment of rheumatoid arthritis and wide range of autoimmune diseases, showing the ability of modulation for inflammation; evidence favors the notion that the endogenous anti-inflammatory autocoid adenosine mediates the anti-inflammatory effects of methotrexate [14]. Intrathecal methotrexate has been considered for salvage immunotherapy in autoimmune encephalitis patients who failed to respond to first-line immunotherapy. However, although intrathecal injection of methotrexate may be reasonable with consideration of the poor penetration of the immune modulators such as intravenous immunoglobulin, steroids, and rituximab across the blood-brain barrier, methotrexate-induced neurotoxicity via intrathecal injection is still a concerning issue [15]. Thus, future studies are needed to confirm the efficacy and safety of the maintenance of oral methotrexate in combination with infliximab treatment. This case report provides evidence of the potential efficacy of infliximab with maintenance of methotrexate in certain cases of refractory cryptogenic brainstem encephalitis.
Notes
Ethics statement
This study was approved by the Research Ethics Committee of Pusan National University Yangsan Hospital, and Informed consent was obtained from the patient to publish her case.
Conflict of interest
Dr. SH Ahn is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Funding
This study was supported by a 2019 research grant from Pusan National University Yangsan Hospital.
Author contributions
Conceptualization: SHA, SKB, JHS. Data curation: KNW, HSK. Formal analysis: SHA, KNW, HSK, JHS. Funding acquisition: SHA. Methodology: JR, SKB. Project administration: JHS. Visualization: JR, SKB. Writing–original draft: SHA. Writing–review & editing: all authors.
