Cerebral hyperperfusion syndrome after endovascular stent graft reconstruction for postirradiated carotid blowout syndrome: a case report
Article information
Abstract
Background
Cerebral hyperperfusion syndrome (CHS) is a failure of autoregulation after a revascularization procedure. It has rarely been reported in patients with no pre-existing cerebral hypoperfusion.
Case Report
We present a rare case of a patient who underwent stent graft implantation to treat postirradiated carotid blowout syndrome. The patient developed hypertension, focal neurological deficit, and seizures after the procedure; neuroimages revealed ipsilateral cerebral edema, swelling, and increased cerebral perfusion. CHS was diagnosed based on clinical and radiological findings. The patient recovered gradually after receiving supportive care.
Conclusion
Owing to the risk of CHS, monitoring for cerebral perfusion and prevention of hypertension is suggested for patients who undergo stent graft placement for postirradiated carotid blowout syndrome.
INTRODUCTION
Cerebral hyperperfusion syndrome (CHS) is an uncommon complication experienced by patients who have undergone revascularization for neurovascular disorders. CHS is most often reported in patients who have undergone carotid endarterectomy or carotid artery stenting, although it has also been reported in patients who have undergone other intracranial procedures [1]. Impaired cerebral autoregulation due to long-standing chronic ischemia is the most commonly reported mechanism underlying this condition. The occurrence of CHS in patients without an underlying chronic neurovascular steno-occlusive disease has rarely been reported. We report a case of CHS after endovascular stent grafting of the extracranial carotid artery for postirradiated carotid blowout syndrome (CBS).
CASE REPORT
A 66-year-old male patient presented with bloody discharge from a left neck wound for 3 days. The patient had a history of nasopharyngeal cancer diagnosed 16 years prior and had received concurrent chemoradiotherapy and left neck dissection for the initial and recurrent disease. The patient’s neck was heavily treated. He received 70 Gy in the left upper neck, 50 Gy in the right upper neck, and 116 Gy in the bilateral lower neck. Neoplastic disease was reported to be stable for the preceding 11 years. However, extensive radiation necrosis of the neck soft tissue occurred 6 years prior to the patient’s presentation at our clinic. Twelve months before presentation, the patient underwent total laryngectomy, esophagectomy, C6 vertebral corpectomy, and pectoralis major myocutaneous flap reconstruction owing to pharyngocutaneous fistula and deep neck infection. Poor healing and infection of the patient’s chronic neck wound developed several times thereafter. The patient was admitted to our hospital for antibiotic therapy.
Sudden-onset massive left neck wound bleeding occurred 3 days after admission. An otolaryngologist on duty immediately applied epinephrine gauze packing to the wound. On examination, high blood pressure (212/130 mmHg) was noted. Emergency computed tomography angiography (CTA) revealed a pseudoaneurysm in the left distal common carotid artery (Fig. 1). No obvious stenosis was observed in the left extracranial carotid or cerebral arteries. Endovascular treatment with a stent graft (8 mm×10 cm) from the internal carotid artery to the common carotid artery (VIABAHN Endoprosthesis; Gore & Associates, Flagstaff, AZ, USA), along with external carotid artery coil embolization, was performed immediately after CTA (Fig. 2). The procedures were performed under local anesthesia. No further angioplasty was performed, and no antiplatelet agents were prescribed before or immediately after the procedure. Neck bleeding subsided thereafter. Eleven hours after stent graft implantation, the patient exhibited right-hand clonic movement and right-leg clenching toes, followed by two episodes of tonic-clonic convulsive seizures. The patient’s blood pressure was 151/103 mmHg. Consciousness disturbance (Glasgow coma scale E3M4V2 status) was noted after seizures. Non-contrast computed tomography (CT) revealed left-hemispheric cortical swelling (Fig. 3A). CT perfusion showed elevated cerebral blood flow and cerebral blood volume in the left cerebral hemisphere compared to that in the right hemisphere (Fig. 3B and C). The stent graft was patent, and no intracranial large-vessel occlusion was observed. Levetiracetam (300 mg) was administered intravenously. The patient’s systolic blood pressure was maintained at <140 mmHg. In the following 2 days, his consciousness level recovered to the baseline condition. Magnetic resonance imaging (MRI) revealed left frontal and parietal subcortical white matter with abnormal hyperintense signals on fluid-attenuated inversion recovery/T2-weighted images (Fig. 3D) 6 days post-seizure activity. No abnormal hyperintense signals were observed on diffusion-weighted imaging (Fig. 3E). The patient was followed-up in the clinic and was able to ambulate after rehabilitation.
Left carotid blowout syndrome diagnosed using preoperative computed tomography angiography. (A) Axial computed tomography (CT) revealing irregular focal protrusion of the left common carotid artery (arrow). (B) Sagittal reformatted CT depicting the location of a pseudoaneurysm (arrow) in relation to the surrounding free flap. No obvious stenosis noted in the carotid artery.

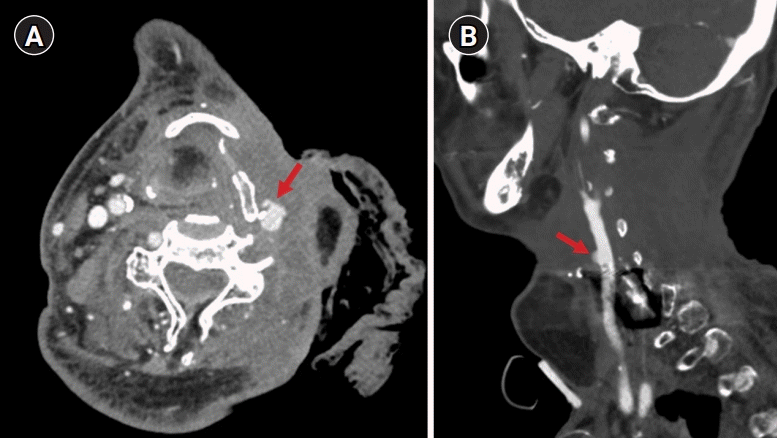
Endovascular treatment for carotid blowout syndrome. (A) Lateral view of the left common carotid revealing a pseudoaneurysm of the left distal common carotid artery (black arrow) corresponding to the computed tomography angiography finding. (B, C) After placement of a stent graft (white arrows) and coils (white arrowhead), the pseudoaneurysm was obliterated. The diameter of the carotid artery did not change considerably after the procedure.

Postoperative neuroimaging findings. (A) Non-contrast computed tomography (CT) revealing substantial sulcal effacement at the left frontoparietal lobe, suggestive of cerebral swelling. No frank hypodensity or hemorrhage is observed. (B, C) CT perfusion revealing elevated cerebral blood flow (B) and cerebral blood volume (C) of the left posterior frontal and parietal lobe, and CT angiography revealing dilatation of the left convexity leptomeningeal arteries compared to those in the contralateral right hemisphere. (D, E) Magnetic resonance imaging revealed subcortical hyperintensity on the fluid-attenuated inversion recovery image but not on the diffusion-weighted image, suggestive of vasogenic edema.
DISCUSSION
CBS is a life-threatening condition requiring emergency management [2]. Endovascular treatment, including both destructive and reconstructive methods, is the current mainstay of CBS treatment [3]. Destructive methods, namely those causing damage to the involved perforated carotid artery, are typically more secure than reconstructive methods but are associated with a higher risk of immediate postoperative ischemic stroke. Employing reconstructive methods involving stent graft exclusion from the ruptured site is an alternative approach to achieving short-term hemostasis. Reported stent graft complications include recurrent hemorrhage, thromboembolism, and brain abscesses [4]. One major concern of the immediate post-procedural period is thromboembolic risk, especially when antiplatelet therapy is not administered in hemorrhagic conditions. Standard post-procedural care usually includes monitoring for these complications, prudent hydration, avoidance of hypotension, and antibiotic use. In patients with CBS, stent grafts are usually deployed in vessels without underlying stenosis, while angioplasty is reserved for endoleaks. Therefore, in addition to neurological examination, cerebral perfusion is not routinely monitored. Nevertheless, the patients’ presentation quickly raises suspicions of CHS, considering their temporal relation to stent graft implantation.
Conceptually, the diagnosis of CHS is based on elevation of cerebral blood flow in the affected territory. CHS is commonly diagnosed based on clinical and radiological features because there is no consensus on the diagnostic criteria for CHS [1]. Headache, elevated blood pressure, seizure, and focal neurological signs are characteristic findings of anterior circulation CHS, which our patient experienced. In addition, immediate CTA revealed leptomeningeal arterial dilatation, and subsequent MRI revealed subcortical white matter edema without frank infarction. At the time of diagnosis, our patient’s presentation was consistent with the clinical features of CHS, and other differential diagnoses were excluded. After supportive care, including antihypertensive control, the patient gradually recovered from neurological symptoms.
Although impaired cerebral autoregulation is the mechanism most commonly presumed to underlie the pathophysiology of CHS, other theories have been proposed. Injury from free radicals, baroreflex breakdown, and abnormal trigeminovascular reflux may also play a role in the development of CHS [5-7]. Previous literature has suggested that baroreflex failure can result from irradiation [8]. In our study, because the patient might have experienced irradiation-associated baroreflex failure, the adaptation to the radial force of the stent graft might have been impaired. During and after the procedure, we did not observe overt hypotension or bradycardia, which commonly occur in patients who have undergone carotid artery stenting. Paradoxically, the patient’s blood pressure persistently increased after the procedure. There have been case reports of CHS without pre-existing significant stenosis in patients undergoing aneurysm coiling [9]. Hirai et al. [10] reported a case of CHS after carotid stent grafting for a giant extracranial internal carotid artery aneurysm. They performed spectrophotometric measurements during the procedure and used CT perfusion to monitor the patient’s cerebral perfusion status postoperatively. However, preoperative ipsilateral cerebral hypoperfusion was observed, suggesting the need for an aggressive surveillance strategy. Hence, this case report suggests that CHS can develop in patients with radiation-associated carotid disease but without critical stenosis.
CHS can occur after stent graft placement in the postirradiated neck without luminal stenosis. Practitioners must be familiar with the presentation and diagnosis of CHS and should carefully monitor the cerebral perfusion and systemic blood pressure of patients with this condition.
Notes
Ethics statement
This study was approved by the Research Ethics Committee of the National Taiwan University Hospital (No. RIND9561703046). The requirement for written informed consent was waived.
Conflict of interest
No potential conflict of interest relevant to this article.
Author contributions
Conceptualization: YHL. Data curation: CWL, TWL. Formal analysis: YCH. Methodology: CWL, YCH, TWL. Visualization: YHL. Writing–original draft: YCH. Writing–review & editing: YCH, YHL.
