A Case of Leuprolide-Induced Posterior Reversible Encephalopathy Syndrome
Article information
Abstract
Background
Leuprolide is a gonadotropin releasing hormone agonist used for patients with prostate cancer and a variety of gynecologic diseases, such as uterine myoma, endometriosis, and adenomyosis. Reported side effects include a local skin eruptions, anaphylactoid reactions, ischemic heart diseases, and seizures.
Case Report
A 41-year-old woman showed the sudden onset of headache. She had a history of being treated by leuprolide for adenomyosis. Her vital sign was stable. Laboratory findings were within a normal range. Right homonymous quadrantanoptic scotoma was observed on perimetry. Her brain magnetic resonance imaging (MRI) showed high signal lesions on T2-weighted (T2WI) and fluid attenuated inversion recovery images affecting left occipital cortex. The MRI results were compatible with the features of posterior reversible encephalopathy syndrome (PRES).
Conclusion
To the best of our knowledge, this is the first case to report a possible association between PRES and the usage of leuprolide.
INTRODUCTION
Leuprolide is a gonadotropin releasing hormone (GnRH) agonist that desensitizes GnRH receptor. Accordingly, Leuprolide decreases the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Also, Leuprolide suppresses production of gonadal sex hormone. Leuprolide is used to treat various diseases, such as prostate cancer, uterine myoma, endometriosis, and central precocious puberty [1]. Adverse effects from the use of leuprolide have been reported. They range from local skin irritation to severe anaphylactoid reactions [2]. Related to cardiac adverse effects, several cases of angina and ischemic heart diseases have been reported from the patients with the history of being treated with leuprolide [3]. Several cases of seizures by leuprolide have been reported and considered as catamenial seizures [4]. On the other hand, cerebral structural lesions caused by leuprolide have not been reported yet. The current study presents the case of posterior reversible encephalopathy syndrome (PRES) that is likely to be induced by the use of leuprolide.
CASE REPORT
A 41-year-old woman was admitted to the Neurology Department due to the sudden onset headache. She used to have mild headache intermittently. The attack was the most severe headache in her life (visual analog scale 9 points). She woke up with the blunting headache. Prior to the attack, she had been diagnosed with adenomyosis that required hysterectomy. After the surgery, she was treated with leuprolide acetate 3.75 mg subcutaneously twice, 4 weeks apart, for relieving symptoms. The last injection was conducted 2 weeks before the headache began. She was a nonsmoker and a nondrinker. She had no other underlying diseases. Her vital sign was stable. Her blood pressure was within a normal range. Laboratory tests revealed no abnormalities. Initial neurological examinations revealed no focal neurological deficits. Right homonymous quadrantanoptic scotoma on perimetry was noticed (Fig. 1). Chest radiography and electrocardiogram showed no abnormal findings. Her brain CT scan revealed hypodensity at the left occipital lobe. Her brain magnetic resonance imaging (MRI) after admission showed multiple lesions with high signal intensity in both occipital, mainly involving cortex and subcortical white matter on T2 and fluid attenuated inversion recovery image. The lesions appeared as high signal intensities on diffusion weighted image (DWI) and apparent diffusion coefficient (ADC) map (Fig. 2). The MR findings were compatible with those of PRES. Injection of leuprolide discontinued. Her headache was gradually alleviated over the 4-month follow-up period. Her brain MRI conducted 4 months after the discharge showed that the previous lesion was partially resolved (Fig. 3).
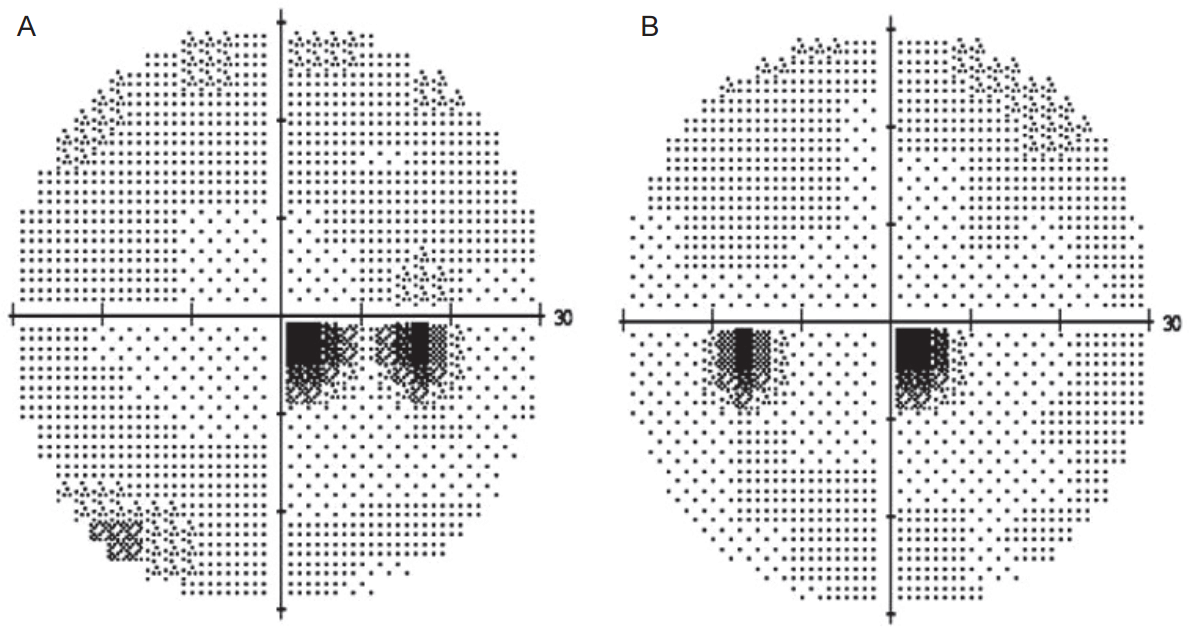
Perimetry (A, Right eye; B, Left eye) shows right homonymous quadrantanoptic scotoma due to a lesion of the tip of the left occipital lobe.
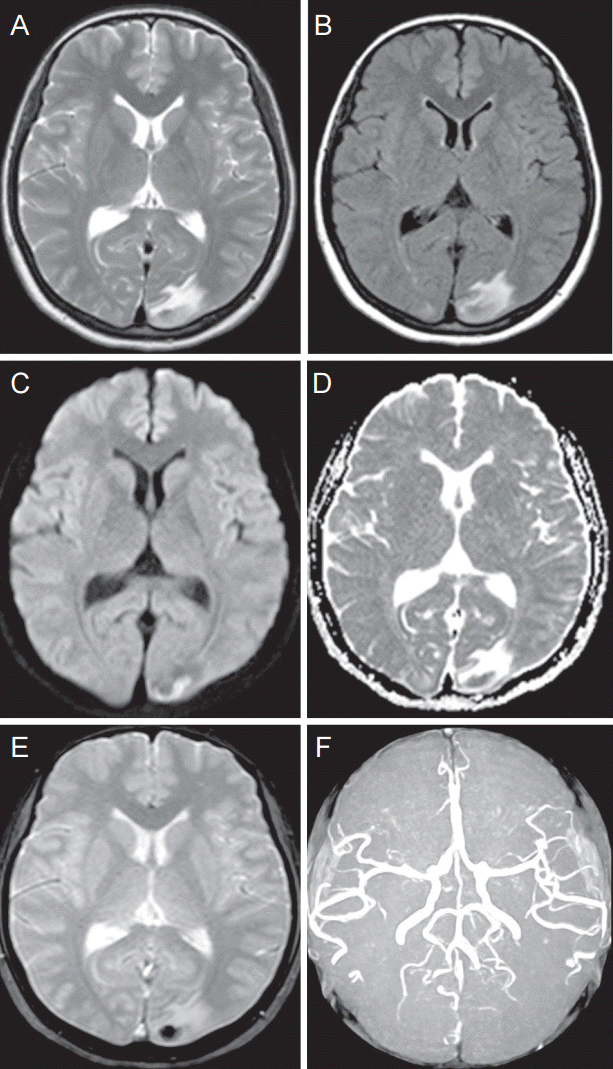
Initial MR images show abnormal high signal change in the left occipital lobe on T2 weighted image (A) and bilateral occipital lobe, predominantly left side, signal change on FLAIR sequence (B). DWI reveals hypointense signal in the left occipital lobe surrounding central hemorrhagic lesion (C). ADC map demonstrates high signal, suggesting vasogenic edema, surrounding central hemorrhagic area (D). GRE shows a focal hemorrhage (E). Normal intracranial angiographywith no evidence of steno-occlusion in posterior cerebral arteries (F). MR, magnetic resonance; FLAIR, fluid attenuated inversion recovery; DWI, Diffusion-weighted image, ADC, apparent diffusion coffficient, GRE, gradient echo image.
DISCUSSION
PRES is defined as a clinicoradiological entity characterized by neurologic symptoms, which include headache, vomiting, altered mental status, blurred vision, and seizures. Typically, cranial MR images suggest vasogenic edema which mainly involve posterior regions of the brain [5]. The important aspect of PRES is reversibility shown in follow-up MR images. The present patient was diagnosed with PRES, according to the characteristics of the MRI finding, partial recovery of MRI lesion, and clinical symptoms.
PRES is known to be related to a hypertensive state of any etiology that include pre-eclampsia/eclampsia [6]. In fact, a significant number of patients appear to be normotensive. Clinical investigations report that PRES can develop in normotensive patients who have been treated with cytotoxic and immunosuppressive drugs under the settings of cancer chemotherapy, allogenic bone marrow or stem cell transplantation for hematologic malignancies, and solid organ transplantation [7]. The association of PRES with various autoimmune diseases, infection, and sepsis has been noticed.
The present case does not have explicit causes for PRES, other than the history of being treated with leuprolide; accordingly, we attribute the development of PRES to direct cytotoxicity of leuprolide and/or endothelial damage related to a hypogonadal state induced by leuprolide. Differential diagnosis of cerebral venous sinus thrombosis was also considered. The patient did not have predisposing prothrombotic conditions with the typical MR features of PRES; thus, we concluded that the patient had PRES, rather than cerebral venous thrombosis.
The pathogenesis of PRES is under debate. Several hypotheses exist [5]. One theory puts emphasis on hyperperfusion. Systemic hypertension with failed autoregulation could lead to forced dilatation of otherwise constricted arterioles. A subsequent injury of the capillary beds and extravasation of serum could result in break-through vasogenic edema. In another theory, hypoperfusion accounts for the pathogenesis of PRES. Vasoconstriction upon evolving systemic hypertension could result in an ischemic change, a blood brain barrier destruction, and vasogenic edema. The previous theory is further supported by the presence of watershed distribution, concurrent infarcted lesion, and petechial hemorrhages in subsets of PRES cases. Lastly, an endothelial damage caused by drugs or general medical conditions would upregulate endothelial surface antigen and loosen brain vessel tight junctions [8]. The role of leuprolide in the development of PRES can be explained either by direct cytotoxicity of leuprolide on the vascular endothelium as do immunosuppressive and cytotoxic drugs, or by a decreased level of estrogen that is known to have protective effects on endothelial cells [9].
Ischemic heart diseases are well documented in patients treated with leuprolide [3]. Since estrogen is known to have cardioprotective effects, hypogonadism induced by GnRH agonist explains the prevalence of ischemic heart diseases in otherwise healthy premenopausal women with no other risk factors [10]. Since ischemic heart diseases and cerebrovascular accidents share common risk factors, it is reasonable to infer that leuprolide might play a role in the development of PRES as well.
In conclusion, leuprolide can be a rare cause of PRES. Therefore, clinical suspicion of PRES should be raised when new onset of headache, visual disturbance, or seizure occurs in patients who have a history of treatment with leuprolide.
