Stroke Recurrence in a Patient Twelve Years after Repair of a Secundum Atrial Septal Defect
Article information
Abstract
Background
Secundum atrial septal defect (ASD) is a common congenital heart defect in adults. Patients with ASDs at high risk of cardiovascular complications undergo either surgical repair or percutaneous device closure.
Case Report
We report the case of an 85-year-old male with unusual recurrent cerebral infarctions. The patient has undergone repair of secundum ASD 12 years ago. Evaluation by transesophageal echocardiography revealed a mobile mass at the patch repair site in the left atrium. The mass was surgically removed due to recurrent stroke during the anticoagulation.
Conclusion
This case emphasizes the importance of regular cardiac checkup and the need to consider cardioembolic source as being part of the etiology of stroke recurrence, even if the event occurs many years after intracardiac shunt closures.
INTRODUCTION
Secundum atrial septal defect (ASD) is a common congenital heart defect in adults. Although most patients with ASDs have a benign prognosis, the European Society of Cardiology guidelines recommend ASD closure for subjects with hemodynamically unstable ASD to reduce long-term complications such as heart failure, stroke, and death [1,2].
Surgical or transcatheter closures can be performed for treatment of patients with ASD who are at a high risk for cardiovascular complications. Surgical closures have been performed for many decades with good long-term prognoses; however, even though successful surgical closures may be performed on patients with ASDs, there remains a risk for cardiovascular complications such as strokes or transient ischemic attacks (TIAs) [3,4]. Left atrial thrombus formation is a characteristic of a neurological complications, which typically occurs within 2 years following a procedure [5]. Evidence from the literature shows that only a few patients experienced neurological complications following an intracardiac shunt procedure.
To the best of our knowledge, our case report is the first evidence of recurrent strokes following a procedure. In the present study, we report a case of an 85-year-old patient with recurrent cerebral infarctions, which first occurred 12 years after surgical repair of secundum ASD.
CASE REPORT
An 85-year-old man was admitted to the emergency department due to sudden onset of visual disturbances. The patient reported having had high blood pressure for 30 years. Coronary artery disease and secundum ASD had been surgically corrected 12 years prior to his visit by coronary artery bypass graft surgery and patch repair, respectively. A diagnosis was done using transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) as imaging tools. Prior to the surgery, the patient had preserved systolic function (ejection fraction [EF]=51%) and no evidence of an intracardiac mass. The patient began taking 100 mg aspirin after the operation and was regularly followed up using TTE and an electrocardiogram.
The last TTE was performed 4 years and showed an intact ASD patch repair site, with no interval change in septal motion, compared with TTE taken after the surgical closure. The initial neurological examination revealed only the presence of left upper quadrianopsia with no other neurological deficits. Based on the National Institutes of Health Stroke Scale score, his stroke severity was scored as 1.
The initial brain diffusion-weighted magnetic resonance imaging (DW-MRI) showed multiple acute cerebral infarctions on the right occipital lobe, which correlated with his visual symptoms (Fig. 1A). The lesions were not enhanced by gadolinium on T1-weighted images and showed no evidence of any hemorrhagic changes on gradient-echo (GRE) images. Brain magnetic resonance angiography showed a mild atherosclerotic change of the right distal posterior cerebral artery (Fig. 1B, Supplementary Fig. 1). To investigate the cardioembolic source, TTE, 24-hour Holter monitoring, and blood workup, including D-dimer and brain natriuretic peptide tests were performed on the patients. Although TTE showed a preserved systolic function (EF=52%), with a slightly aggregated regional wall motion abnormality at the right coronary artery territory, no evidence of the cardiogenic source was found. Since there was no significant stenosis and a cardiac source of the embolism, we considered the mechanism of cerebral infarction as being of undetermined etiology. Upper quadrianopsia persisted in the patient as disease sequelae and was discharged with a prescription of dual antiplatelet agents (aspirin, clopidogrel).
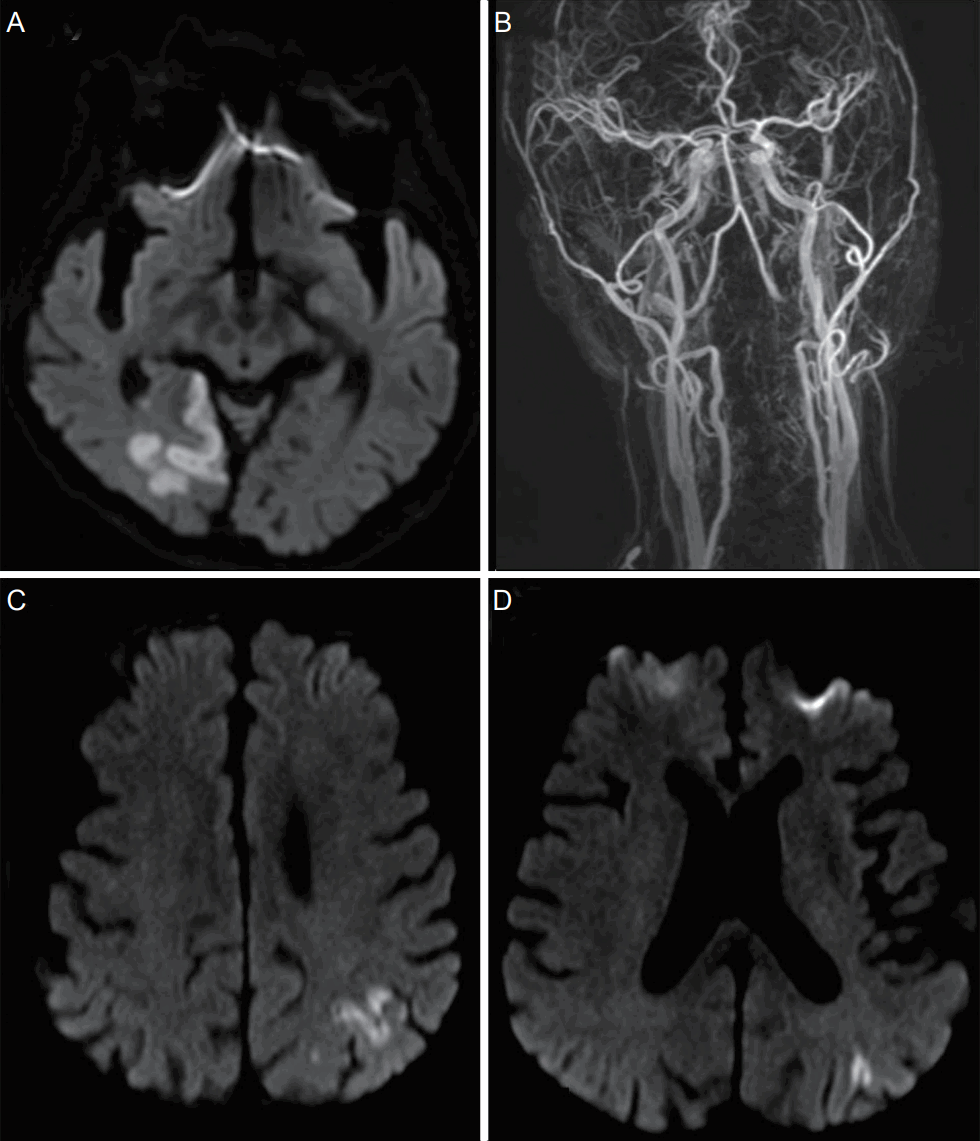
Brain MRI of recurrent cerebral infarctions and contrast enhanced MRA. (A) Diffusion weighted MRI shows acute infarction on the right occipital lobe. (B) MRA shows no significant stenosis on bilateral proximal internal carotid and vertebral arteries. (C) First follow up diffusion weighted MRI shows acute cerebral infarction on the left parietal lobe. (D) Second follow up diffusion weighted MRI shows acute infarction on the left parieto-occipital lobe. MRI, magnetic resonance image; MRA, magnetic resonance angiography.
Two weeks after discharge, the patient was readmitted due sudden onset of right arm weakness, hypoesthesia, and dysarthria. Brain DW-MRI showed an acute cerebral infarction on the left parietal lobe (Fig. 1C). Because the new cerebral infarction occurred on the opposite side, with no evidence of significant arterial stenosis, TEE was performed to further evaluate the cardiac source of the embolism. TTE showed a 0.89×0.42 cm-sized hypermobile mass at the patch repair site in the left atrium (Fig. 2). Warfarin was started as an anticoagulant due to initial impression of the mass as a thrombus. The patient was discharged with no additional sequelae.
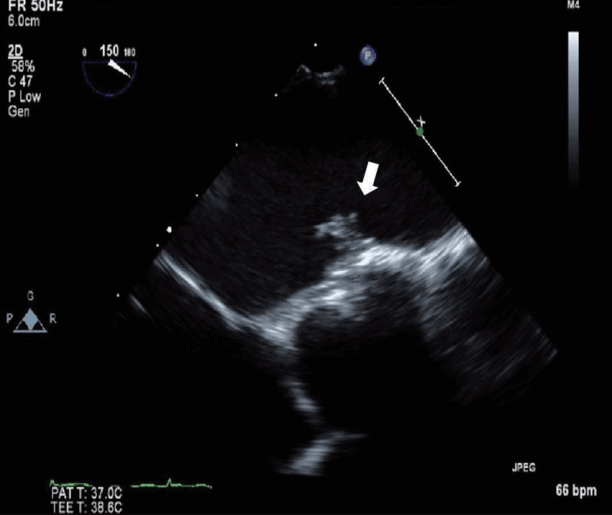
Transesophageal echocardiography shows hypermobile amorphous echogenic mass (0.89×0.42 cm, arrow) attached to the patch repair site of atrial septal defect in the left atrium.
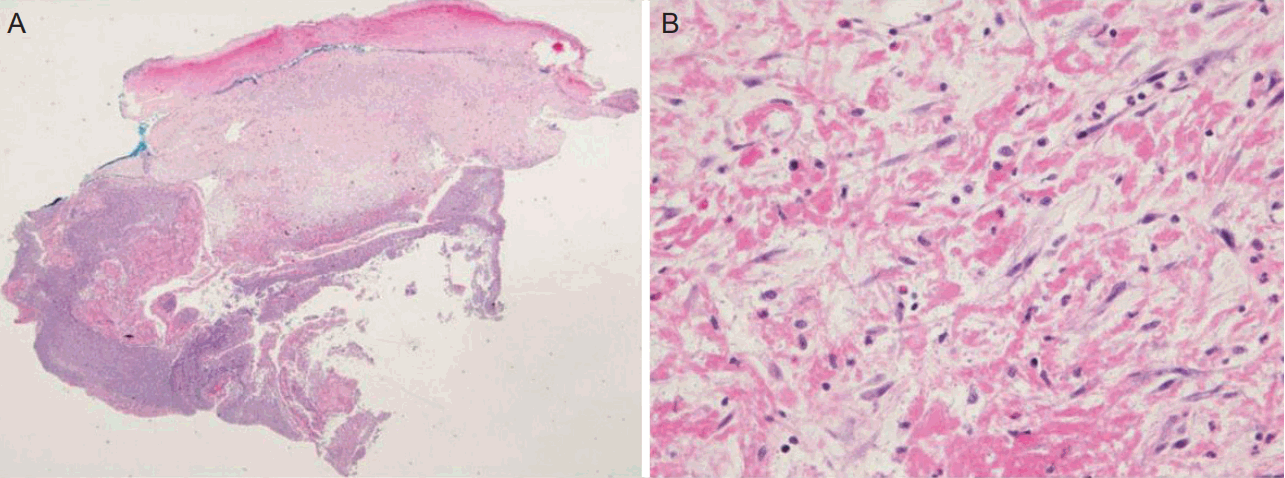
However, 2 weeks after anticoagulation therapy, the patient presented with transient right arm and leg weakness. The symptoms lasted for one hour and could be described as follows: clinically a presumptive diagnosis of a TIA was made, but brain DW-MRI showed another new left parieto-occipital lobe infarction, with no evidence of hemorrhagic change on GRE (Fig. 1D, Supplementary Fig. 2). The international normalized ratio was 2.79. A follow-up TEE was performed and showed a mobile mass at the patch repair site in the left atrium. Open-heart surgery was performed, since anticoagulation treatment alone did not reduce the size of the mass. Surgeons found severe fibrosis around the previous ASD patch and an inflammatory fibrous pus-like mass, which appeared to be evidence of infection. However, prior to performing the surgical procedure, there was no signs of infection. White blood cell counts, C-reactive protein, erythrocyte sedimentation rate, and body temperature were normal. Tissue culture and pathological investigation of the removed surgical patch and hypermobile mass showed no bacterial growth or thrombus in the mass (Fig. 3). With no evidence of infectious endocarditis based on clinical presentation, laboratory examination, and pathological investigation, the patient was discharged with an antiplatelet agent after the surgery.
DISCUSSION
The incidence rates of strokes in surgically-closed ASD reportedly ranged from 0.57-2.9% [5,6]. Homma et al. [7] reported three TIAs and one ischemic stroke among 532 patients, occurring over a time period of 1-13 months after surgical patent foramen ovale (PFO) closure.
Most of the strokes that occur after ASD closure are due to atrial thrombi. The incidence of thrombus formation after ASD closure using percutaneous devices ranges from 2.5-27% [8]. The most frequent neurological events occur within the first 12 months, and only a few cases of recurrent strokes are reported to have occurred 5 years or longer after device closure of intracardiac shunt [9-11].
Currently, most of the intracardiac shunts, regardless of ASD or PFO, are performed using percutaneous devices, as in the above-mentioned cases. The unique feature of the present case is that ASD closure was performed surgically and stroke recurrence occurred three times over a time period that was more prolonged than described in previous reports.
Initially, we did not find a mass at the patch repair site using TTE and found a hypermobile mass after recurrent infarction using TEE. The mass was not reduced in size by adequate anticoagulation treatment, and a stroke recurred during administration of the anticoagulants. The exact etiology of the recurrent strokes is unclear. A thrombus formed, probably due to an inflammatory fibrotic change of the patch site. Although this is a speculative cause, the thrombus disappeared following intensive anticoagulation therapy. Another possibility is that a portion of the pus-like mass might have been the source of the infarction.
TEE was not performed during the 12 years of follow-up after ASD closure. Therefore, we have no knowledge of when the intracardiac mass was formed. The exact etiology of the recurrent cerebral infarction is unknown; therefore, to the best of our knowledge, this is the first report of a delayed recurrent stroke after the repair of ASD. This case emphasizes the importance of regular cardiac checkups and the need to consider cardioembolic source as being part of the etiology of a stroke recurrence, even if the event occurs many years after intracardiac shunt closures.
Supplementary Materials
The online-only supplementary material is available with this article at https://doi.org/10.18700/jnc.180037.
Time of flight magnetic resonance angiography of (A) anterior circulation and (B) posterior circulation shows mild stenosis of right posterior cerebral artery (arrow).
Diffusion-weighted magnetic resonance images of (A) 2nd ischemic stroke and (B) 3rd ischemic stroke show new acute cerebral infarction on left parieto-occipital lobe (arrow).
Acknowledgements
This study was presented at 8th Korea-Japan Joint Stroke Conference on October 20, 2017.