Feasibility, Safety, and Follow-up Angiographic Results of Endovascular Treatment for Non-Selected Ruptured Intracranial Aneurysms Under Local Anesthesia with Conscious Sedation
Article information
Abstract
Background
At most centers, general anesthesia (GA) has been preferred for endovascular treatment (EVT) of ruptured intracranial aneurysms (RIAs). In this study, we analyzed procedural results, clinical outcomes, and follow-up angiographic findings for patients undergoing EVT for RIA under local anesthesia (LA) with conscious sedation (CS).
Methods
We retrospectively evaluated 308 consecutive patients who underwent EVT for RIAs at a single institution between June 2009 and February 2017. EVT under LA with CS was considered for all patients with aneurysmal subarachnoid hemorrhage, regardless of Hunt and Hess (HH) scale score.
Results
EVT was performed for 320 aneurysms in 308 patients with subarachnoid hemorrhages. The mean patient age was 55.5±12.6 years. Moderate (III) and poor (IV, V) HH grades were observed in 75 (24.4%) and 77 patients (25%), respectively. Complete occlusion immediately after EVT was achieved for 270 (84.4%) of 320 aneurysms. Thromboembolic complications and intraprocedural ruptures occurred in 25 (7.8%) and 14 cases (4.3%), respectively. The morbidity rate at discharge (as defined by a modified Rankin scale score of 3 or greater) was 27.3% (84/308), while the mortality rate was 11.7% (36/308). Follow-up angiographic results were available for 210 (68.1%) of 308 patients. Recanalization was observed in 64 (29.3%) of 218 aneurysms in 210 patients.
Conclusion
Based on our experience, EVT for RIAs under LA with CS was feasible, regardless of the clinical grade of the subarachnoid hemorrhage. Complication rates and follow-up angiographic results were also comparable to those observed when GA was used to perform the procedure.
INTRODUCTION
Since the International Subarachnoid Aneurysm Trial demonstrated its safety and efficacy, endovascular treatment (EVT) involving coil embolization has been a mainstay of treatment for both unruptured and ruptured intracranial aneurysms [1]. At most centers, coil embolization for ruptured intracranial aneurysm (RIA) is performed under general anesthesia (GA), which provides optimal conditions for the procedure [2]. However, GA does not allow the evaluation of neurological status during the procedure, and several complications are associated with mechanical ventilation and anesthetics [3].
At our institution, we performed EVT under local anesthesia (LA) with conscious sedation (CS) for all patients with RIA, regardless of the clinical severity of the subarachnoid hemorrhage (SAH). However, few studies have evaluated the procedural results of patients treated in this manner [4,5]. Furthermore, the long-term follow-up angiographic results of RIAs treated by EVT under LA with CS have not been reported. Therefore, in the present study, we evaluated the procedural results, clinical outcomes, and long-term follow-up angiographic findings of patients undergoing EVT for RIAs under LA with CS.
METHODS
Patients and data collection
We retrospectively evaluated findings from 308 consecutive patients with aneurysmal SAHs who underwent EVT at a single institution between June 2009 and February 2017. This study was approved by the appropriate Institutional Review Board (IRB no. 05-2018-176), which waived the requirement for informed consent due to the retrospective nature of the study. Patients’ demographics, initial neurological severity, length of hospital stay, clinical outcome at discharge, and mortality were extracted from each patient’s electronic medical records. At the time of EVT for RIA, the following initial angiographic data were also collected: location and size of the treated aneurysms, height-to-neck ratio of the aneurysms, and immediate angiographic results following the procedure.
Anesthetic technique
EVT under LA with CS was considered for all patients with aneurysmal SAH, regardless of the Hunt and Hess (HH) scale score. The groin was infiltrated with 5 mL of 1% lidocaine. A low dose of intravenous remifentanil (0.02-0.1 μg/kg/min) was administered continuously for CS during the procedure. Ramsay sedation scale was used for assessment of the level of sedation [6]. Light sedation of Ramsay sedation scale at 2 or 3 was used to avoid respiratory depression, snoring, and maintain patient cooperation for neurologic evaluation. An additional bolus of low-dose of propofol or midazolam was administered if the procedure could not be continued due to patient immobility and agitation. Patients who were expected to exhibit unstable respiration were intubated for airway protection prior to the procedure and additional mechanical ventilation was applied to patients with respiratory failure due to initial neurologic severity. However, an anesthesiologist did not attend the administration of anesthetics, and inhaled anesthetics and muscle relaxant were not used, except for the continuous infusion of low-dose remifentanil and an additional bolus of midazolam or propofol, when necessary. These procedures allowed for neurologic examination of patients undergoing mechanical ventilation. We defined the procedure as being performed under LA with CS, according to whether or not neurologic examination was possible, regardless of the application of mechanical ventilation. We continuously monitored patients’ vital signs, including blood pressure, heart rate, respiratory rate, oxygen saturation, and electrocardiography.
Endovascular procedure
One neuroradiologist and one neurosurgeon performed each procedure. A radiolucent head-fixation device attached to the angiographic table was used to reinforce the immobility of the patient’s head. A bolus of intravenous heparin (50 IU/kg) was injected immediately after placement of the guiding catheter. Under fluoroscopic guidance, aneurysms were treated in accord with standard procedures, using appropriate microcatheters, microwires, coils, and stents, as indicated.
Angiographic follow-up
First and second follow-up radiologic examinations were performed using conventional catheter angiography at 6 months and 1.5 years after the initial procedure. If the second examination showed no recanalization or no change of minor recanalization, a third follow-up angiography was performed using magnetic resonance angiography (MRA) one year after the second follow-up. If conventional angiography was not feasible, the exams were performed using MRA with three-dimensional reconstruction. The angiographic outcomes were determined based on Raymond-Roy occlusion classification [7].
Statistical analysis
Statistical analyses were performed with SPSS version 21.0 software for window (SPSS Inc., Chicago, IL, USA). Univariate analysis using Fisher’s exact test, independent sample t-test, and Pearson’s χ2 test were used, as appropriate. Multivariate logistic regression was performed to assess the independent contributions of HH grade to morbidity, mortality, and the degree of aneurysm obliteration. The results were considered significant for P values <0.05.
RESULTS
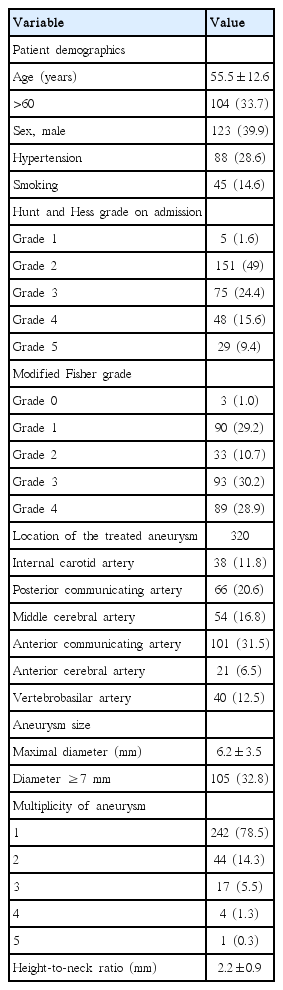
Characteristics of patients and aneurysms
During the study period, a total of 308 consecutive patients harboring 399 aneurysms who had presented with aneurysmal SAH were treated via EVT. The study sample was composed of 123 men (39.9%) and 185 women (60.1%). The mean patient age was 55.5±12.6 (standard deviation) years. A total of 104 patients (33.7%) were age 61 or older. Eighty-eight patients (28.6%) exhibited hypertension, while 45 (14.6%) were smokers. Mild (I, II), moderate (III), and severe (IV, V) HH grades were noted in 156 (50.6%), 75 (24.4%), and 77 patients (25%), respectively. Sixty-six patients (21.4%) had multiple aneurysms, and 12 patients were treated for two aneurysms at the same time. Of the 320 aneurysms treated, most lesions involved the anterior (n=280, 87.5%), rather than the posterior circulation (n=40, 12.5%). The aneurysms were located in the following vessels: internal carotid artery (n=38, 11.8%), posterior communicating artery (n=66, 20.6%), middle cerebral artery (n=54, 16.8%), anterior communicating artery (n=101, 31.5%), anterior cerebral artery (n=21, 6.5%), and vertebrobasilar system (n=40, 12.5%). The mean aneurysm size was 6.2±3.5 mm and a total of 105 (32.8%) large aneurysms (7 mm or greater) were observed. Mean height-to-neck ratio of the aneurysms was 2.2±0.9. Table 1 shows the characteristics of patients and aneurysms.
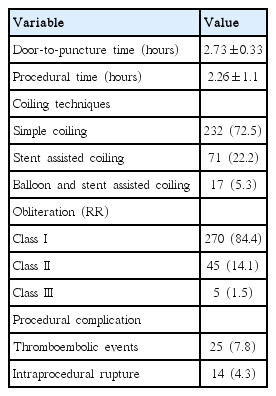
Procedural results, complications, and clinical outcomes
The median door-to-puncture time was 2.73±0.33 hours, while mean procedure time was 2.26±1.1 hours. Simple coil embolization (CE), stent-assisted CE, balloon and stent-assisted CE were performed for 232 (72.5%), 71 (22.2%), and 17 aneurysms (5.3%), respectively. Complete occlusion (Raymond-Roy classification I) immediately after EVT was achieved for 270 (84.4%) of 320 aneurysms (Table 2). Procedure-related adverse events involving thromboembolic complications and intraprocedural ruptures occurred in 39 (12.1%) cases, 25 (7.8%) and 14 (4.3%), respectively.
Among the 308 included patients, EVT was completed under our protocol of anesthesia for 307 patients, while the remaining patient had extreme agitation and required conversion to general anesthesia with muscle relaxant and inhaled anesthetics under the supervision of an anesthesiologist. Seventy-three patients (23.7%) were intubated prior to the procedure and mechanical ventilation was applied for 34 patients (11.0%). Two patients of HH grade 3 underwent tracheal intubation and mechanical ventilation due to respiratory depression related CS during the procedures; however, the procedures were completed successfully. The morbidity rate at discharge (as defined by a modified Rankin scale score of 3 or greater) was 27.3% (84/308), while the mortality rate was 11.7% (36/308).
Follow-up angiographic results
Follow-up angiography results were available for 218 aneurysms of 210 (68.1%) patients. Conventional catheter angiography was used for most of these patients (conventional angiography: n=204, 97.1%; MRA only: n=6, 2.9%). The mean time to last angiographic follow-up was 18.4 months. Recanalization was observed in 64 (29.3%) of 218 aneurysms in 210 patients (Table 3). Twenty-eight of these aneurysms were retreated due to major recanalization. Adequate occlusion immediately after retreatment was achieved for 27 of 28 aneurysms.
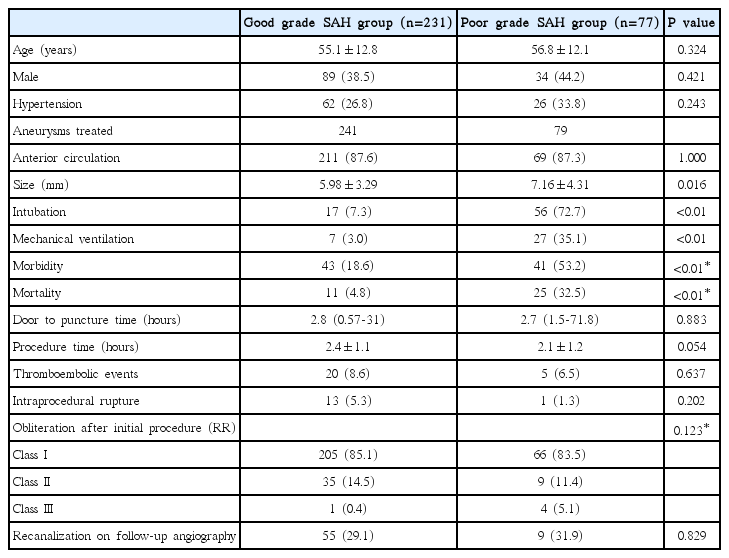
Results according to HH grades
Good grade SAH group and poor grade SAH group were defined by HH grades 1 to 3 and 4 to 5, respectively. Clinical outcomes and procedural results between the good grade SAH group and the poor grade SAH group were compared (Table 4). The rates of tracheal intubation and application of mechanical ventilation were significantly higher in the poor grade SAH group than in the good grade SAH group (intubation rate: 72.7% versus 7.3%, odds ratio [OR] 34.0, 95% confidence interval [CI] 16.3-70.3, P<0.001; rate of application of mechanical ventilation: 35.1% versus 3.0%, OR 16.0, 95% CI 6.6-39.2, P<0.001). The mean size of aneurysms was significantly larger in the poor grade SAH group than in the good grade SAH group (7.16±4.31 mm versus 5.98±3.29 mm, P=0.016). The rates of morbidity and mortality were also significantly higher in the poor grade SAH group than in the good grade SAH group (morbidity: 53.2% versus 18.6%, P<0.001; mortality: 32.5% versus 4.8%, P<0.001). However, there was no significant differences in procedural complications, obliteration degree of aneurysms after initial procedure, or recanalization rate of follow-up angiography between the two groups.
DISCUSSION
In most centers, EVT for RIAs has been preferred under GA, which allows for airway protection, the maintenance of patient immobility for high image quality, and prevents sudden unexpected complications related to patient motion during the procedure [2]. However, LA allows for neurological evaluation during the procedure, shortens the procedure room time, and reduces complications that occur under general anesthesia and mechanical ventilation, such as cardiopulmonary morbidity and mortality [3,8,9]. In a systematic review and meta-analysis of 1,956 patients treated with LA or GA anesthesia types, Brinjikji et al. [8] reported that patients receiving general anesthesia had higher odds of mortality (OR 2.59, 95% CI 1.87-3.58, P<0.01), and respiratory complications (OR 2.09, 95% CI 1.36-3.23, P<0.01). Several studies have demonstrated that placement of a flow diverter and EVT for unruptured intracranial aneurysms can be performed safely under LA [10,11]. Ogilvy et al. [11] reported a high rate of successful procedure completion (74.6%) and low rates of overall procedure-related morbidity (1.2%) and mortality (0.6%), for a total of 496 unruptured aneurysms treated by LA with CS. In this study, even for non-selected RIAs, although mechanical ventilation was applied to 34 patients (11.0%), procedures were completed for most (307) of the 308 patients under CS, demonstrating the feasibility of EVT under LA with CS. Furthermore, to the best of our knowledge, this is the first large-scale study to present the long-term follow-up angiographic results of non-selected RIAs treated by EVT under LA with CS.
EVT may lead to procedure-related complications. Thromboembolic complications and intraprocedural perforation for intracranial aneurysms, including unruptured aneurysms under GA have been reported to occur in 11-12.5% and 2.5-5% of cases, respectively [12-15]. A previous study reported the rates of thromboembolic complications (7.7%) and intraprocedural rupture (3.8%) among patients with good grade SAH receiving CS [4]. Park et al. [5] reported a high rate of successful completion (98.4%) of RIAs treated by EVT under LA, but the intraoperative rupture rate (12.9%) was significantly higher for LA than GA, although thromboembolic rates (12.4%) were similar. This study showed a complication rate of 12.1% among patients with non-selected RIA, which included rates of thromboembolic events and intraprocedural perforation of 7.8% and 4.3%, respectively. There were also no significant differences in procedural complications, obliteration degree of aneurysms after the initial procedure, and recanalization rate seen at follow-up angiography between the good grade SAH group and the poor grade SAH group. However, the rates of tracheal intubation and application of mechanical ventilation were significantly higher in the poor grade SAH group than in the good grade SAH group. Therefore, airway protection may be required for patients with poor grade SAH, even if the procedures are performed under LA with CS.
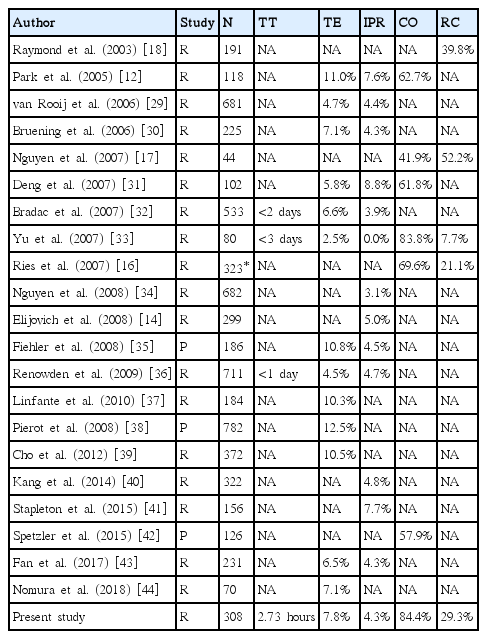
A major concern associated with EVT for RIA is the risk of recanalization of a coiled aneurysm, which potentially re-bleeds. Previous studies have reported various rates of recanalization following EVT for intracranial aneurysms, including unruptured aneurysms under GA, ranging from 7.7% to 52.2% [16-18]. Several studies have reported that RIA is a risk factor for the recanalization of coiled aneurysms [17,18]. In this study, despite follow-up angiographic data collected primarily through conventional catheter angiography, the recanalization rate was 29.3%.
Re-bleeding is known to occur more frequently in the earlier phase after SAH, with the highest frequency occurring within the first 24 hours (15%), especially within the first 3-6 hours [19,20]. Previous studies advocated for treatment of aneurysms as early as possible after SAH because the risk of poor outcome increased as time-to-treatment after SAH increased [21-24]. In our study, EVT was initiated at a median of 2.73 hours after patients had arrived at the emergency room, and the mean procedure time was 2.26 hours. Early EVT was possible because there was no time required to prepare for GA, and it also shortened the total room time for the procedure.
Thus, our outcomes were comparable to the results of the above series performed under GA, as well as other studies under LA. Table 5 shows the summary of literature reports on EVT for RIA under GA.
Remifentanil is an ultra-short acting opioid which is quickly metabolized and does not accumulate in the tissue [25]. Continuous infusion of remifentanil with predictable pharmacokinetics has been used to achieve CS during various surgical procedures [26-28]. However, its continuous infusion can also cause respiratory depression and inhibition of the sympathetic nervous system, resulting in decreased heart rate and blood pressure [25]. A previous report demonstrated a higher incidence of respiratory depression in the CS group receiving remifentanil at an infusion rate of 0.1 μg/kg/min, compared to an infusion rate 0.05 μg/kg/min [28]. In this study, EVT for 89% of patients was completed without mechanical ventilation. However, severe respiratory depression occurred in two cases of CS and required urgent intubation during the procedures. These results highlight the caution that must be employed using LA with CS.
This study has several limitations. This study was a retrospective study performed at a single institution. As such, our results are susceptible to selection bias and the effects of an incomplete dataset. Furthermore, adverse events were self-reported, and we did not compare outcomes between those treated under LA and those treated under GA. Although light sedation of the Ramsay sedation scale at 2 or 3 was desired for appropriate CS, there is a possibility that a considerable number of patients were overly- sedated by infusion of sedative drugs. LA may not be suitable for patients who are extremely restless and are deemed too risky to be treated under this type of anesthesia. Nevertheless, we experienced the feasibility of EVT under LA with CS for non-selected RIAs, and we hope that a future large prospective multicenter trial will verify our findings.
CONCLUSION
LA does not allow for high-quality radiographic imaging during EVT, and patients may experience discomfort and pain during the procedure. However, based on our experience, EVT for non-selected RIAs under LA with CS is feasible, and the rates of procedural complication and immediate, follow-up angiographic results were also comparable with those observed following the use of GA.