Targeted temperature management for postcardiac arrest syndrome
Article information
Abstract
Neurocritical care management to improve neurologic outcome for postcardiac arrest syndrome (PCAS) has focused considerably on targeted temperature management (TTM). TTM attenuates the destructive processes following ischemia/reperfusion in PCAS. The principal indication of TTM is a patient with sustained coma after return of spontaneous circulation (ROSC). TTM can be strongly recommended with a target temperature between 32°C and 36°C for patients with shockable rhythm and out-of-hospital cardiac arrest (OHCA) and weakly recommended for patients with initial asystole or pulseless electrical activity with OHCA and those with in-hospital cardiac arrest. TTM is induced and maintained using a cooling device with body temperature feedback under appropriate analgosedation. It requires the intensive management of various systemic respiratory, circulatory, and metabolic parameters that control shivering to prevent secondary brain damage. Considering the cerebral perfusion pressure, it is suggested that the mean arterial pressure should be particularly maintained over 80 mm Hg. Seizure management, including continuous electroencephalography monitoring, is also needed. Finally, we must continue the above mentioned care during and after the rewarming phase, because high fever and shivering may appear again during this period. Furthermore, neurological prognostication should be performed at least 72 hours after ROSC through clinical investigations and multimodal testing without sedation.
INTRODUCTION
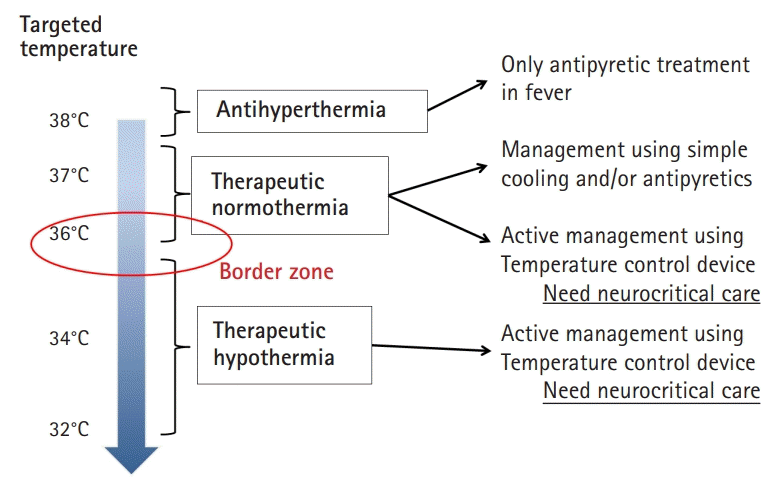
Targeted temperature management (TTM) is a clinical treatment strategy to control core body temperature (target temperature) for a certain duration to reduce secondary brain injury. Fig. 1 shows the general definition of antihyperthermia, therapeutic normothermia, and therapeutic hypothermia. Generally, therapeutic hypothermia is defined as a core body temperature of 32°C to 34°C (TTM 32°C to 34°C) using various methods. It is important to know that therapeutic normothermia, which involves maintaining a core body temperature of 36°C to 37°C (TTM 36°C to 37°C) using various methods, is different from no cooling or normothermia. Therefore, it is more appropriate to use the terms TTM 32°C to 34°C or TTM 36°C instead of therapeutic hypothermia or normothermia, respectively.
The destructive processes following ischemia/reperfusion in postcardiac arrest syndrome (PCAS) are divided into primary injury and secondary injury [1,2]. Primary injury begins immediately after cardiac arrest and is caused by cessation of cerebral blood flow (CBF). As cerebral oxygen delivery decreases, adenosine triphosphate (ATP) production stops, causing energy-dependent ion pump dysfunction. Intracellular Na+ accumulation results in cytotoxic edema, and depletion of ATP leads to anaerobic metabolism, cerebral lactate accumulation, and intracellular acidosis. Cellular ischemia causes influx of Ca2+ into cells, which activates lytic enzymes and mitochondrial injury, further depleting ATP. Excitatory neurotransmitter release activates lipases and proteases, causing apoptosis.
Secondary injury begins immediately after return of spontaneous circulation (ROSC) and takes place in the hours and days following cardiac arrest. After ROSC, reperfusion injury causes neuronal damage despite restoration of cerebral oxygen delivery. An initial period of cerebral hyperemia is followed by hypoperfusion, resulting in a “no-reflow” state that exacerbates secondary injury. The reason for “no-reflow” is microcirculatory dysfunction and/or impaired vasomotor regulation caused by microthrombi, decreased nitric oxide production, and increased intravascular viscosity due to extravasation of intravascular water (blood brain barrier disruption). Free radical release, glutamate production, and intracellular Ca2+ accumulation also leads to reperfusion injury and/or microcirculatory dysfunction.
These primary and secondary mechanisms are all stimulated by fever. Many observational studies have reported that fever after ROSC was related to poor neurological outcomes [3,4]. In the no control of TTM scenario, almost all patients will develop a fever early after ROSC [5,6]. Both mechanisms are also inhibited by hypothermia. Reduction in core temperature decreases the cerebral metabolic rate of oxygen (CMRo2) and attenuates several intracellular pathways involved in secondary brain damage which occur in the minutes and days after collapse [1]. For TTM, active temperature control, shivering prevention, and intensive care unit (ICU) bundle care are needed, regardless of the selected targeted temperature. TTM to prevent fever is reasonable for neuroprotection.
EVIDENCE FOR TTM
For out-of-hospital cardiac arrest patients with shockable rhythm
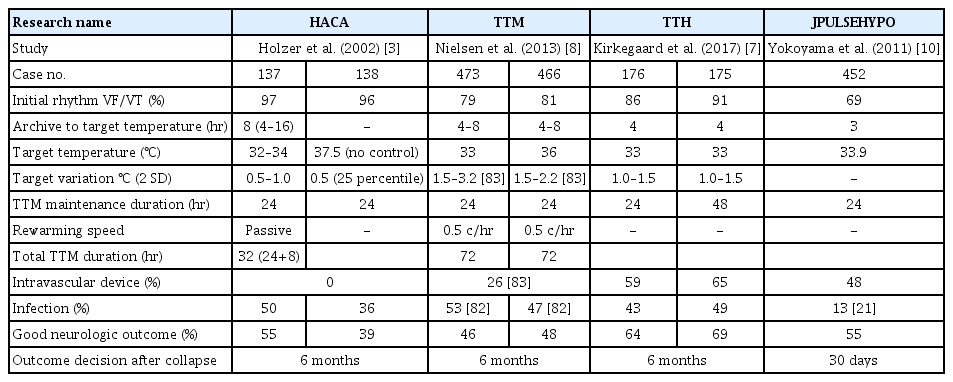
TTM significantly improved neurologic outcomes in patients with PCAS of suspected cardiac origin who were treated under bundle care (Table 1) [5-7]. In 2002, a landmark study on TTM was published, which indicated that therapeutic hypothermia (TTM 32°C to 34°C, 24 hours cooling and 8 hours rewarming) resulted in better neurological outcomes at 6 months when compared to the outcomes without fever control in patients with out-of-hospital cardiac arrest (OHCA) due to an initial shockable rhythm [5]. In the same year, Bernard et al. [6] also showed that therapeutic hypothermia (TTM 33°C, 12 hours cooling and 6 hours rewarming) increased the proportion of patients with OHCA due to an initial shockable rhythm and of patients who could return home or participate in rehabilitation at discharge when compared to the outcomes without fever control. These two studies allowed managing physicians to make better decisions regarding prognostication and withdrawal of life-sustaining therapies. Another landmark study on TTM (the TTM trial) published in 2013 showed that TTM (36°C, 24 hours, followed by 8 hours of rewarming to 37°C and temperature maintenance below 37.5°C until 72 hours) was as effective (in terms of primary outcome and mortality) as therapeutic hypothermia (32°C to 34°C) and is an acceptable alternative to it [8]. Nevertheless, it is important to confirm the findings of the TTM trial, in which 80% of patients had ventricular fibrillation (VF)/ventricular tachycardia (VT) and 20% did not have VF/VT (pulseless electrical activity [PEA]/asystole), as the severity of brain injury might have been high (Table 1). In the TTM trial, providing a defined prognostication protocol resulted in a longer observation period. Lopez-de-Sa et al. [9] compared the temperatures of 32°C and 34°C for therapeutic hypothermia (24 hours) and reported that there was no significant difference in patient independence at 6 months. A multicenter registry in Japan enrolled 452 adult patients (shockable rhythm, 68.9%) undergoing therapeutic hypothermia (33.9℃±0.4℃) and showed that the proportion of patients with favorable neurologic outcome was 55.3% at 30 days after cardiac arrest [10]. These data support the use of temperature control. The International Liaison Committee on Resuscitation (ILCOR), American Heart Association (AHA), and American Academy of Neurology (AAN) recommend TTM at a target temperature between 32°C to 36°C for patients with OHCA due to shockable rhythm [11-13].
Although Deye et al. [14] reported that a target temperature between 32% to 34°C remained unchanged for 56% respondents in 2016 after TTM trial, the use of therapeutic hypothermia decreased in a United States registry of patients with OHCA reported in 2018 [15].
For OHCA patients with nonshockable rhythm
Regarding a nonshockable rhythm (PEA/asystole), the TTM trial presented data showing that there was no significant difference in death rates between patients who underwent therapeutic hypothermia (32°C to 34°C) or no therapeutic hypothermia (36°C) [8]. Other studies show an association between therapeutic hypothermia and favorable outcome [16-20] or survival [16,18]. In Japan, Soga et al. [21] reported that post-ROSC cooling is an effective treatment for patients with nonshockable cardiac arrest when the time interval from collapse to ROSC is short. TTM 32°C to 36°C for patients with initial asystole or PEA is also supported by the AHA, ILCOR, and AAN guideline [11-13]. Regarding TTM for nonshockable rhythm PCAS, the first large randomized control trial was published recently (the therapeutic hypothermia after cardiac arrest in nonshockable rhythm (HYPERION) trial) [22]. In this French trial, 581 adult patients who were comatose after resuscitation from either an in-hospital cardiac arrest (IHCA) or OHCA with an initial nonshockable rhythm were randomized to either TTM 33°C or TTM 37°C, both for 24 hours. At 90 days, 29 of 284 patients (10.2%) in the 33°C group were alive with a cerebral performance category of 1 or 2, as compared with 17 of 297 (5.7%) in the normothermia group (risk difference, 4.5%; 95% confidence interval, 0.1 to 8.9; P=0.04). This trial reinforces the recommendation of considering TTM 32°C to 36°C for PCAS patients with nonshockable rhythm. Patients with nonshockable rhythm tend to have numerous noncardiac issues and higher mortality than those in VF/VT [23]. Further studies are needed to determine the role of TTM in this patient population.
For IHCA patients
For patients with IHCA, the Guidelines-Resuscitation database suggested poor outcome (regarding survival to hospital discharge and neurologic outcome) with TTM [24]. A potential selection bias, however, should be pointed out while interpreting this data. According to the current guidelines, TTM should be considered for patients with IHCA [11-13]. We must also await further studies.
INCLUSION AND EXCLUSION CRITERIA FOR TTM
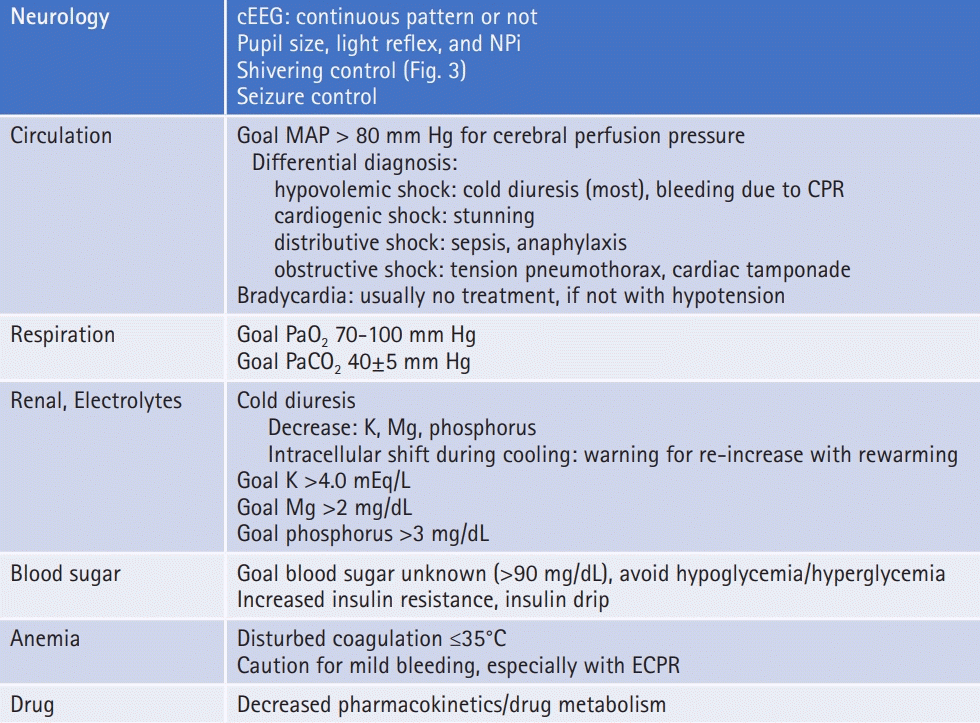
TTM is recommended for adult patients in a coma (Glasgow Coma Scale [GCS] ≤8 and E=1 and V=1 or 2 and M≤5) after ROSC, irrespective of an initial cardiac rhythm, although patients with OHCA due to a shockable rhythm are expected to show better outcomes than those with nonshockable rhythm (Fig. 2). TTM is also considered to be performed in patients with OHCA due to nonshockable rhythm or in patients with IHCA. The most important consideration in a TTM operation is adequate neurocritical care with TTM.
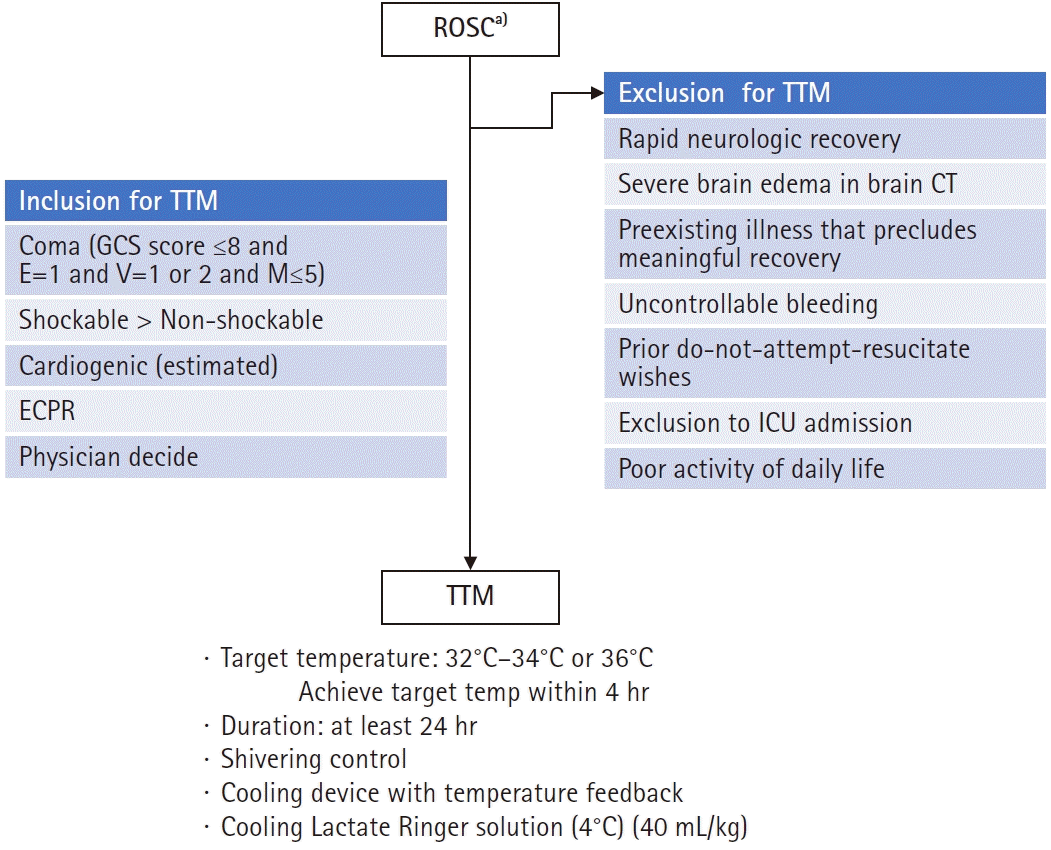
Special considerations before and during targeted temperature management (TTM) induction. ROSC, return of spontaneous circulation; GCS, Glasgow Coma Scale; ECPR, extracorporeal cardiopulmonary resuscitation; CT, computed tomography; ICU, intensive care unit. a)Coronary angiography with percutaneous coronary intervention for ST-elevated myocardial infarction.
If patient consciousness level has recovered rapidly recovered and they are able to follow verbal commands (GCS motor score=6), TTM is not recommended. Although a patient with GCS motor score 5 is reported to not be a candidate for TTM in one study [25], further research is needed to determine whether GCS motor score 5 is a suitable threshold for patients to not be candidates for TTM. TTM is not indicated for patients who have a preexisting illness that precludes meaningful recovery or those considering a do-not-resuscitate order. Other contraindications are shown in Fig. 2. Finally, if the time interval between cardiac arrest and TTM initiation is long, the neuroprotective effect of TTM may be limited. Practically, this means that TTM is not applied for cardiac arrest patients 12 hours after collapse [26].
TEMPERATURE SELECTION: CONSIDERING 36°C
Therapeutic hypothermia (TTM 32°C to 34°C), which protects secondary brain injury more as compared to TTM 36°C, may produce coagulopathy and bleeding. If the patient has surgical bleeding, intracranial bleeding, hemorrhagic diathesis, or trauma, TTM 36°C should be considered, because it usually does not cause coagulopathy. Finally, TTM 36°C using an intravascular cooling device can be performed in the rare cases of patients who have cold agglutinins (usually activated only below 31°C).
For circulatory management, compared to TTM 36°C, TTM 32°C to 34°C may result in more hemodynamic instability [27] that may require vasopressor support.
INDUCTION OF TTM
For patients eligible for TTM, tracheal intubation and monitoring of several aspects, including circulation, respiration, and metabolism, should be performed. Core body temperature is monitored using a bladder, endovascular, or esophageal probe.
If the patient is eligible for TTM, it must be induced and target temperature must be reached as soon as possible (Fig. 2). Table 1 shows longer time intervals to achieve targeted temperature, especially in Hypothermia after Cardiac Arrest Study Group study [5], possibly because cooling devices with temperature feedback were not used. Stanger et al. [28] reported that initiation of TTM (door-to-TTM) within 122 minutes of hospital admission was associated with improved survival. Care must be taken to control the time interval between collapse and ROSC, because this interval might determine the outcome.
In patients with cardiac failure, TTM should be induced under extracorporeal cardiopulmonary resuscitation (ECPR) which can manage core body temperature. If the patient does not have accompanying left ventricular dysfunction, TTM is induced using a temperature control device (endovascular, surface, intranasal, or esophageal cooling) with automated feedback temperature control through continuous input of the patient’s core temperature [29]. Recent studies show rapid achievement of target temperatures using temperature control devices and shivering management.
Rapid infusion of cold (4°C) lactated Ringer infusion (40 mL/kg) decreases the core body temperature by 1°C for each liter [30,31] in the emergency room and/or ICU. However, prehospital use of cold fluids increases the risk of rearrest and pulmonary edema [32]. Shivering control and analgosedation is needed for rapid induction of TTM, regardless of targeted temperature with/without ECPR.
EXTRACORPOREAL CARDIOPULMONARY RESUSCITATION
Among patients without ROSC but with good neurological indicators (shockable rhythm, short time interval between collapse and ROSC), ECPR is an option for resuscitation. Ortega-Deballon et al. [33] reviewed several cohort studies and reported that the overall survival rate with ECPR was 20% among patients without ROSC; however, the survival rate is known to vary among studies. In Japan, the Study of Advanced Cardiac Life Support for Ventricular Fibrillation with Extracorporeal Circulation in Japan (SAVE-J) study reported that among OHCA patients with a shockable rhythm on initial electrocardiography, the rate of good neurological outcomes (11.2%) at 6 months after insult was higher with treatment including ECPR, therapeutic hypothermia, and intraaortic balloon pump (IABP) than with treatment not involving ECPR (2.6%) [34]. Thus, ECPR is a good approach for resuscitation in selected patients.
One study revealed that IABP with percutaneous coronary intervention (PCI) contributed to improved neurologic outcome under cardiogenic shock after ROSC [35]. Another study revealed that ECPR in addition to PCI improved neurologic outcome in patients who failed to respond to conventional CPR if the collapse-to-bypass interval was less than 55.5 minutes [36]. IABP or ECPR may be considered in patients for whom the cause of cardiac arrest is suspected to be reversible. Furthermore, cardiogenic shock should not be a reason to avoid TTM, and TTM before and/or during acute PCI is not a contraindication. Practically, if TTM has already been started before PCI, possible complications (low blood pressure and/or hypopotassemia due to massive urination, etc.) must be checked and treated even in catheter laboratory (see heading: circulatory care, electrolyte management).
TREATMENT FOR MYOCARDIAL DYSFUNCTION
Acute coronary syndrome is a common cause of cardiac arrest, and treatment for revascularization is necessary. In patients with ST-segment elevation or left bundle branch block on initial electrocardiography after ROSC, the prevalence of an acute coronary lesion is more than 80% [37]. If the cause of cardiac arrest is ST-elevation acute myocardial infarction (STEMI), immediate coronary angiography (CAG) with/without PCI is recommended. Even for non-STEMI that induces cardiac arrest, CAG with/without PCI is recommended, because prehospital electrocardiography does not identify an occluded coronary artery [38], and a previous study found that 25% of patients with non-STEMI had an occluded coronary artery [39]. Although Lemkes et al. [40] recently reported that a strategy involving immediate CAG was not found to be better than delayed CAG for PCAS patients who had no signs of STEMI, the relatively lower severity of the patients included was pointed out as a research limitation [41]. Further study is needed regarding necessity of emergency CAG.
Reperfusion injury, in addition to ischemic insult, is a main cause of myocardial dysfunction. Many studies indicate that PCI improves survival or neurologic outcome [42,43]. Some studies have demonstrated that better survival and functional outcome is achieved after ROSC with a combination of TTM and PCI for STEMI [44,45]. In one randomized controlled trial and analysis that did not involve cardiac arrest patients, this combination reduced the size of the cardiac muscle ischemic lesion in case hypothermia was achieved before reperfusion of the coronary artery [46,47]. Clinical research reported that TTM (lower core temperature) before PCI can decrease the severity of myocardial infarction and might improve cardiac function after ROSC [48]. Studies of survival or neurologic outcome of emergency PCI for non-STEMI are inconsistent; some investigators did not find it helpful [49], but others reported favorable resolutions [42,43,45,50].
SHIVERING CONTROL
Shivering is a physiologic homeostatic mechanism to maintain body temperature and is usually initiated at approximately 36°C [51]. Shivering is severe at TTM 36°C due to the patient thermoregulatory defenses, which are partly suppressed at 32°C to 33°C [1,30]. One interesting report showed that shivering control and analgosedation use are difficult with TTM at 36°C [52]. Sustained shivering causes an increased metabolic rate and cardiac output, tachycardia, elevated blood pressure, increased lethal cardiac complications, increased carbon dioxide (CO2) production, wound pain, increased CMRo2 and intracranial pressure (ICP) [53], and increased stress response. Shivering commonly occurs during TTM and may lead to failure to achieve or maintain adequate hypothermia. Therefore, the management of shivering, including its evaluation and treatment using adequate analgosedation, is important and necessary during TTM to preserve the cerebral oxygen demand/supply balance and strict temperature management.
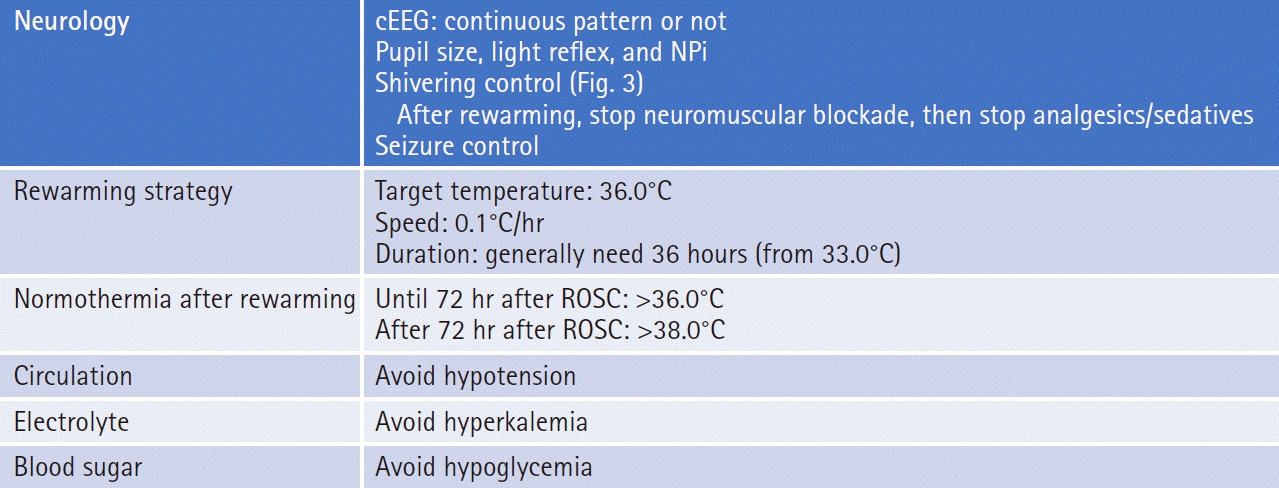
Warning signs of shivering are goose bumps, masseter palpation, electrocardiography artifacts, difficulty in cooling, and temperature increase in spite of TTM. Shivering should be assessed using a subjective, simple, and reliable clinical scale such as the Bedside Shivering Assessment Scale every 1 hour in the ICU [53]. To suppress shivering and prevent prolonged sedation and paralysis, a stepwise antishivering protocol during TTM is recommended [29,54]. However, it does not necessarily need to be stepwise and depends on the intensity of shivering. In general, patients with PCAS who are comatose during TTM need tracheal intubation and therefore require sedation and analgesia. At initiation of hypothermia, skin counter-warming using nonpharmacologic methods should be considered even when surface cooling methods are used for TTM (Fig. 3) [55-57]. This involves the warming of the noncooled areas of the skin (i.e., the face, hands, feet) using a warm-air blanket even when surface cooling methods are used. Drug therapy should include magnesium sulfate, dexmedetomidine, remifentanil, fentanyl, meperidine, and scheduled acetaminophen. If shivering is still not controlled, propofol, midazolam, and finally neuromuscular blockade (NMB) may be initiated [54]. To suppress shivering, a combination of methods should be used, and shivering should be aggressively controlled. It is presumed that the combined use of NMB in addition to complete analgosedation to achieve rapid induction of TTM and NMB titration after achieving targeting temperature are an alternative method.
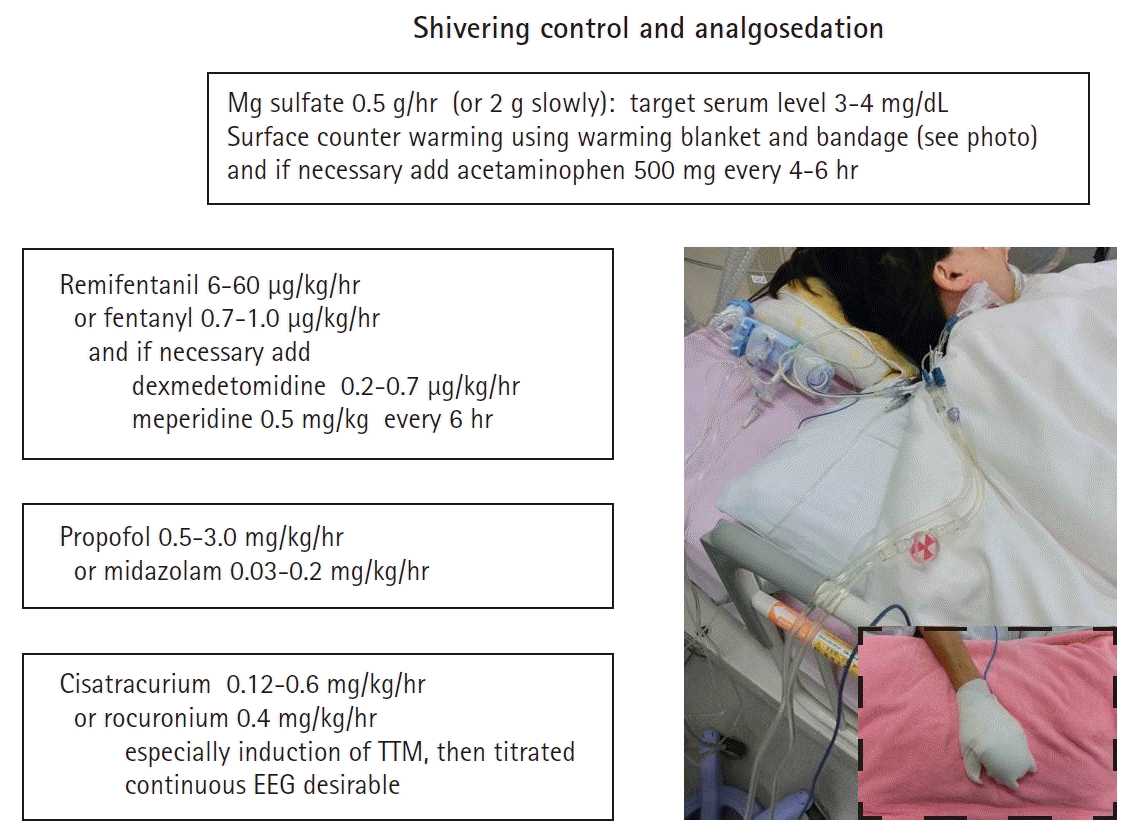
Shivering control and analgosedation. TTM, targeted temperature management; EEG, electroencephalography; BSAS, bedside shivering assessment scale.
On the other hand, one study reported that patients with the most severe brain injuries have less shivering [58]. Other studies have shown that shorter time to target temperature is associated with poor neurologic outcome [59,60]. It is hypothesized that patients with more severe or irreversible neurologic damage are less reactive to low temperatures, so there is less shivering [60] and a reduced requirement for NMB [61]. The relationship between shivering and outcome has yet to be fully elucidated.
ANALGOSEDATION
Sedation may reduce secondary cerebral ischemia and decrease elevated ICP by reducing the CMRo2, CBF, and cerebral blood volume (CBV) [62]. Sedation and analgesia also help control shivering and seizures, which is required for the induction and maintenance of TTM to reduce the risk of brain damage caused by seizures (Fig. 3).
On the other hand, sedation makes it difficult to perform an accurate neurologic examination and clinical assessment. After arrest, residual sedation or paralysis confounds the clinical examination [63]. One study showed that patients undergoing TTM (≤33°C) recovered consciousness in a mean of 3.8 days, with approximately 20% awakening after 5 days postarrest [64]. Recently, Rey et al. [65] reported that increased utilization of midazolam during the TTM phase correlates with late awakening (median time to awakening 5 days; range, 3 to 23 days from sedation stop).
Early interruption of sedation during TTM causes shivering. In brain-injury patients, interruption of sedation causes increased ICP [66]. Therefore, a wake-up test should be avoided during the first 24 hours after ROSC [67]. It is suggested that the tapering of sedative infusions should not exceed 25% per day [51]. In patients at risk of brain edema or who have an elevated ICP, uncontrolled status epilepticus, or ongoing hypothermia, sedatives should not be abruptly discontinued. When weaning of sedation commences, attention should be paid to these risks with appropriate use of such tools as computed tomography, electroencephalography (EEG), and ICP monitoring.
Drugs for TTM
Propofol has a rapid onset and short duration of action which allows for meaningful neurologic examinations [68]. It is associated with a greater risk of hypotension with cerebral hypoperfusion than other drugs, as well as the risk of propofol infusion syndrome. Propofol significantly decreases CBV by causing vasoconstriction [69].
Midazolam results in less hemodynamic instability than propofol, but it prolongs the duration of mechanical ventilation and length of ICU stay [70]; furthermore, it may prolong the time to awakening and reduce the accuracy of the clinical examination, because the half-life of midazolam is prolonged by hypothermia [65,71]. During TTM, low continuous infusions of midazolam are preferred.
Dexmedetomidine is short-acting, provides mild to moderate sedation and analgesic effects, allows clinical assessment, and may be neuroprotective [72]. Dexmedetomidine directly lowers the shivering threshold by central alpha-2 agonism [73]. However, it frequently causes hypotension and bradycardia.
NMB is selectively administered during TTM, resulting in more rapid achievement and maintenance of target temperature and control of shivering [74]. A short-acting NMB can be helpful in patients with refractory shivering who are sedated with continuous propofol/midazolam use. Some older studies suggest that continuous NMB has a beneficial effect and improves outcome [75-77]. Currently, intermittent dosing is preferred to continuous infusions. Continuous NMB infusion was not associated with improved outcomes in one small randomized controlled trial [78]. A multicenter study found that intermittent as needed NMB was associated with improved outcomes when compared to continuous NMB [79]. NMB should be used only if the patients are completely sedated. Moreover, clinicians have to be careful of the risk of pressure injury, deep vein thrombosis, and critical illness polyneuropathy resulting from complete immobilization. NMB masks seizures that are typically detected during the neurological evaluation. EEG monitoring should be considered in comatose patients after cardiac arrest, particularly if NMB is used [12,80].
TTM MAINTENANCE AND DURATION
There are many guidelines and reviews for detailed discussion of the entire course of TTM (Fig. 4) [29,81]. Patients are maintained at the target temperature for at least 24 hours (maintenance phase); different studies have reported a range of durations, from 12 to 48 hours, but 24 hours is generally recommended (Table 1) [29]. Regarding the optimal duration of TTM, Kirkegaard et al. [7] reported that there was no significant difference in 6-month survival between therapeutic hypothermia at 33°C for 24 and 48 hours among patients with combined cardiac rhythm (shockable, 90%). During the maintenance phase, the variation of temperature should be managed to be minimal, because trials have shown that huge variations of temperature (overcooling) might be linked to complications, like infection [8,82,83]. Several studies suggest the association between minimal variation in temperature and a high percentage of favorite neurologic outcomes; they are summarized in Table 1 [7].
SEIZURE MANAGEMENT AND CONTINUOUS EEG
Detection of seizures
Seizures are caused by abnormal excessive or synchronous neuronal activity in the brain [84]. Seizures are not only the result of brain injury caused by cardiac arrest but also a risk for secondary brain injury. They are classified as generalized convulsive seizures or nonconvulsive seizures; muscle contraction and relaxation are absent in the case of nonconvulsive seizures.
The incidence of nonconvulsive status epilepticus (NCSE) in comatose postarrest patients is 12% to 24% in adult PCAS [85-88]; an even higher incidence (47%) has been reported in pediatric cardiac arrest [86]. Other abnormal EEG patterns are found in maximally 40% of patients which are treatable [87]. Based on continuous EEG (cEEG) records, some studies indicate that seizures occur most often within the first 8 hours after ROSC [85,87,88]. Seizures are masked by NMB in 3% to 44% of cases [85,86,89,90]. For these reasons, cEEG monitoring and assessment of NCSE during the induction, maintenance, and rewarming periods are indicated for all TTM patients (Figs. 4, 5) [86,91,92]. Seizures following cardiac arrest are associated with increased mortality [85,87,88].
Prophylactic and therapeutic use of antiepileptic drugs
Some investigations have revealed that antiepileptic drugs (AEDs) do not decrease the incidence of convulsive seizures or improve neurologic outcome [93,94]; furthermore, the effects of AEDs are not standardized. As there is no standard method to diagnose seizures using cEEG and drugs may cause adverse effects (hypotension, etc.), the prophylactic use of AEDs is not recommended. There is no high-grade evidence showing a relationship between AED use and survival or neurologic outcome [95-97], but as seizures may lead to secondary brain injury, treatment of recurrent seizures could be considered as standard therapy in comatose patients with PCAS.
CIRCULATORY CARE
Optimal mean arterial blood pressure and cerebral perfusion pressure
Transient myocardial systolic or diastolic dysfunction [98-101] and a decline in systemic vascular resistance [100] has been observed in PCAS, but may be less clinically significant and can be managed conservatively [101]. One study showed the best survival in patients with a mean arterial blood pressure (MAP) of 76 to 86 mm Hg and mixed venous oxygen saturation of 67% to 72% [102]. Another study reported that a time-weighted average MAP ≥70 mm Hg was associated with a better neurologic outcome than lower levels [103]. MAP ≥100 mm Hg during the 2 hours after ROSC was associated with better neurologic recovery at hospital discharge (retrospectively examined) [104]. Young et al. [105], however, found no relationship between higher MAP during therapeutic hypothermia and neurologically intact survival. Bundled care with goals of MAP of 80 to 100 mm Hg, central venous pressure (CVP) ≥8 mm Hg, and central venous oxygen saturation ≥65% led to better neurologic outcomes and less mortality than in historic controls [106]. A bundle requiring MAP ≥65 to 70 mm Hg, CVP ≥8 to 12 mm Hg, and hemoglobin ≥9 to 10 g/dL showed a better survival rate to hospital discharge and neurologic outcome at 1 year [89].
Regarding cerebral perfusion pressure (CPP) in PCAS patients in a previous study [107], ICP increased to around median 10 mm Hg (interquartile range [IQR], 5 to 20) in patients with good outcomes and to 25 mm Hg (IQR, 10 to 30) in those with poor outcomes. A relatively low burden of intracranial hypertension (ICP >20 mm Hg) was also reported [108,109]. After ROSC, prolonged cerebral hypoperfusion develops within hours and may last for hours to days [110]. During this hypoperfusion, cerebral vascular resistance is increased, and pressure autoregulation is right-shifted or absent, resulting in decreased blood flow oxygen delivery and increased CPP needed to maintain microvascular flow [111,112]. Observational studies show a consistent association between lower postarrest blood pressure and mortality [113,114]. Moreover, maintaining a MAP >80 mm Hg is associated with improved outcomes, even if achieved using a vasopressor [89,106,113,115]. Recently, Sekhon et al. [116] reported that the optimal MAP to prevent brain hypoxia in case series with multimodal neuromonitoring is about 80 mm Hg.
Studies suggest that the MAP should be kept higher than a defined threshold during the postarrest period considering CPP in the damaged brain. Although there is some concern about higher MAP achieved using vasoactive agents and poor outcomes [27], recently, Jakkula et al. [117] reported, based on a multicenter study, that there is no significant difference in neuron-specific enolase concentration at 48 hours after cardiac arrest and neurologic outcome between low-normal (65 to 75 mmHg) and high-normal (80 to 100 mm Hg) MAP management [117]. There is no evidence that a higher MAP causes increased ICP and worsening of outcome. Taken together, it is important to maintain CPP normally, and considering CPP, it is suggested that MAP should be maintained over 80 mm Hg (Fig. 4).
Fluid resuscitation
The amount of fluid required to maintain MAP after arrest was reported to be 3.5 to 6 L [99,100]. However, the relationship between fluid or blood products and outcome after ROSC is unclear, in contrast to what is known about albumin in sepsis [118]. If fluid resuscitation alone is ineffective, it is reasonable to use vasoactive drugs [100,113]. In patients with acute coronary syndrome, emergency CAG with/without PCI should be considered [119].
Heart rate and arrhythmia management
In hypothermia, bradycardia occurs normally and is associated with reduced systolic dysfunction in animal models [120]. A heart rate of 30 to 40 beats/min is common at TTM 33°C and generally does not require therapy unless associated with hypotension [1]. Bradycardia may be more pronounced at lower target temperatures; symptomatic bradycardia may be treated using a beta agonist instead of atropine, which has been found to be ineffective [1].
A lowered heart rate during TTM is considered to be associated with favorable outcomes. A recent study has shown that bradycardia and a low heart rate are predictors of favorable neurological outcomes [121,122]. More recently, a relationship between the heart rate response during rewarming and favorable outcomes has been suggested [123]. In this study, an increased heart rate during rewarming predicted favorable neurological outcomes. The heart rate during TTM is a key indicator of brain variability.
Arrhythmias may develop if the core temperature accidentally falls below 28°C (30°C if electrolyte disorders are present); therefore, the core temperature must be maintained above 30°C. Arrhythmias should not be viewed as a reason to discontinue TTM. QT prolongation is common during TTM, and concomitant QT prolonging drugs should be used with caution [1].
RESPIRATORY CARE
Oxygenation
The cause of hypoxemia in patients with PCAS includes lung contusion induced by chest compressions, atelectasis, ventilator-associated lung injury, and others. It is no wonder that hypoxemia in PCAS may induce secondary brain damage beyond that during the arrest itself because of inadequate cerebral oxygen delivery. Some studies have indicated that hypoxemia after ROSC is associated with worse outcomes than normoxemia [124-126]; therefore, hypoxemia may need to be avoided after ROSC.
Positive end-expiratory pressure (PEEP) is another factor associated with oxygenation. Protective mechanical ventilation with a lower tidal volume and higher PEEP is more commonly used after cardiac arrest. This appears to reduce the incidence of pulmonary complications, although other organs are still at risk [127]. A consensus on PEEP settings for patients with PCAS is lacking, although increasing PEEP may elevate ICP [128]. It may be rational to maintain the PEEP as low as possible as long as higher concentrations of oxygen can be avoided.
Hyperoxemia after ROSC promotes the formation of reactive oxygen species (oxidative stress), which can induce secondary injury in brain tissue already damaged by cardiac arrest. Both observational studies [124,126,129,130] and metaanalyses [131-133] show that hyperoxemia is associated with poor survival and neurologic outcome in PCAS. Although the conclusions of other studies have differed [125,134], it may be necessary to avoid hyperoxemia after ROSC.
It may be concluded that both hypoxia and hyperoxia should be avoided, and a PaO2 of 70 to 100 mm Hg is reasonable (Fig. 4).
Ventilation
Although cerebral pressure autoregulation may be impaired after resuscitation, CO2 reactivity of the cerebral vasculature after ROSC is preserved during mild therapeutic hypothermia [135,136], and therefore, CO2 should be controlled during TTM. Hypocapnia following hyperventilation causes cerebral vasoconstriction and inadequate blood flow, based on some observational studies, and it can certainly cause and/or worsen cerebral ischemia, worsen outcome, and cause injury to other organs in PCAS [137-140].
Increased PaCO2 may cause further worsening of an elevated ICP by increasing the CBF. However, evidence of the effect of hypercapnia on outcome after ROSC is conflicting [134,138-140]. Although a phase II randomized controlled trial found that S100 calcium-binding protein beta concentrations decreased over time in patients with PaCO2 maintained at 50 to 55 mm Hg but not in those with a PaCO2 of 35 to 45 mm Hg and better functional recovery with a PaCO2 of 50 to 55 mm Hg, hospital mortality did not differ significantly between the two groups [141].
Taken together, the risk of poor outcome appears to differ for hypocapnia and hypercapnia, even if PaCO2 deviations from normal are comparable (Fig. 4). Although there is insufficient evidence to recommend routine use of mild hypercapnia after cardiac arrest, hyperventilation should be avoided.
ELECTROLYTE MANAGEMENT AND GLYCEMIC CONTROL
During hypothermia induction, particularly to lower target temperatures, an initial cold diuresis may result in hypokalemia, hypomagnesaemia, and hypophosphatemia. Moreover, hypothermia moves potassium from the extracellular to intracellular space. Frequent assessment of electrolytes and repletion is indicated. Repletion of potassium is carefully done, since serum potassium levels will predictably rise when rewarming is initiated. A target potassium level of 4.0 mmol/L is reasonable during TTM induction and maintenance [29]. Magnesium and phosphorus should be maintained in the high to normal range (Fig. 4).
Glycemic control is important in the management of critically ill patients. The current concepts of glycemic control recommend avoiding hypoglycemia and minimizing glycemic variability (GV). One database study in France showed that smaller magnitudes of GV were observed in patients with a good neurologic outcome compared with those with a poor outcome [142]. Other studies reported that increased GV was associated with increased mortality and unfavorable neurologic outcome [143,144]. This suggests that attention should be paid to GV. In patients with PCAS, the optimal target range remains unknown. However, insulin sensitivity increases and blood glucose levels decrease as body temperature rises during TTM. Further, patients with diabetes may be tolerant to higher glucose values. Blood glucose levels should be checked frequently to avoid hypoglycemia and hyperglycemia (Fig. 4).
REWARMING AFTER TTM
The rewarming phase follows maintenance and should be slow and controlled in order to avoid critical complications. Active normothermia is typically maintained for 24 to 48 hours after rewarming is completed (Fig. 5). Recently, Hifumi et al. [145] reported that a longer rewarming duration was significantly associated with and was as an independent predictor of favorable neurologic outcomes in OHCA patients who received therapeutic hypothermia.
PROGNOSTICATION
Even clinical findings like brain death are not definitive for at least 24 hours following ROSC [146]. In the first 72 hours after cardiac arrest, no sign, symptom, or combination of findings short of brain death precludes favorable recovery [12,147]. As in many neurocritical care conditions, however, accurate neurological prognostication after ROSC is challenging. Pupil diameter and/or pupil light reflex might be associated with brain injury. Recently quantitative pupillometry has allowed a more comprehensive assessment of pupillary function when using pupillary light reflex (PLR) and/or neurological pupil index (NPi). The PLR is expressed as the percentage pupillary constriction in response to a calibrated light stimulus, and PLR <10% is considered as abnormal. The NPi is a scalar value (between 0 and 5, <3 is considered as abnormal) which is calculated based on an algorithm that accounts for several measured pupillary variables, including size, percentage constriction, constriction velocity, dilation velocity, and latency. Oddo et al. [148] reported, based on a multicenter study, that NPi <2.0 at day 1 to 3 after cardiac arrest has an association with poor neurologic outcome (specificity 1.0). Based on earlier estimation of neurologic outcome, Riker et al. [149] reported that 6 hours after ROSC, an NPi <1.5 was associated with poor neurologic outcome (specificity 1.0). In both studies, sensitivity was low. In Japan, Tamura et al. [150] reported that 0 hour after ROSC, the cutoff value of PLR 11% was associated with favorable neurologic outcome (specificity 0.81). Regarding cEEG, Ruijter et al. [151] recently reported that continuous background patterns at 12 hours after cardiac arrest are associated with good recovery (specificity 0.91) and suppression (<10 μV) pattern with poor outcome (specificity 1.0) [151]. Using EEG, poor outcome may be predicted within 24 hours after cardiac arrest under TTM.
Withdrawal of life-sustaining therapy based on perceived neurological prognosis has been linked to preventable deaths after cardiac arrest [152,153]. Early aggressive care must be performed initially.
CONCLUSION
TTM for PCAS is a fundamental strategy in neurocritical care. Further studies are needed to identify the types of cases in which patients would benefit from TTM and to determine the optimal target temperature and duration of TTM.
Notes
Conflict of interest
No potential conflict of interest relevant to this article.
Author contributions
Conceptualization & Writing-original draft: YK. Writing-review editing: KK.