INTRODUCTION
Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) are classified into separate disease entities from multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD) [1,2]. MOGAD manifests as diverse demyelinating syndromes, and unusual manifestations have been reported [1,3]. Although demyelinating events expressed as abnormal thermoregulatory responses in NMOSD or MS are rare, manifestation as central fever in MOGAD is very rare [4,5]. In this study, we report a case of a patient with MOGAD manifesting as central fever.
CASE REPORT
A 56-year-old woman presented with an acute quadriparesis with limb paresthesia 2 days prior to examination. The patient had complained of nausea and vomiting 3 days before. On a neurological examination, the patient was mildly drowsy and complained of dysarthria and dysphagia, and then a nasogastric tube was inserted. A neurological examination revealed a weakness of Medical Research Council grade 4 in the upper extremities and grade 0 to 1 in the lower extremities, with hypesthesia below the L2–L3 sensory dermatome. The patient had been treated for hypertension and diabetes mellitus for several years.
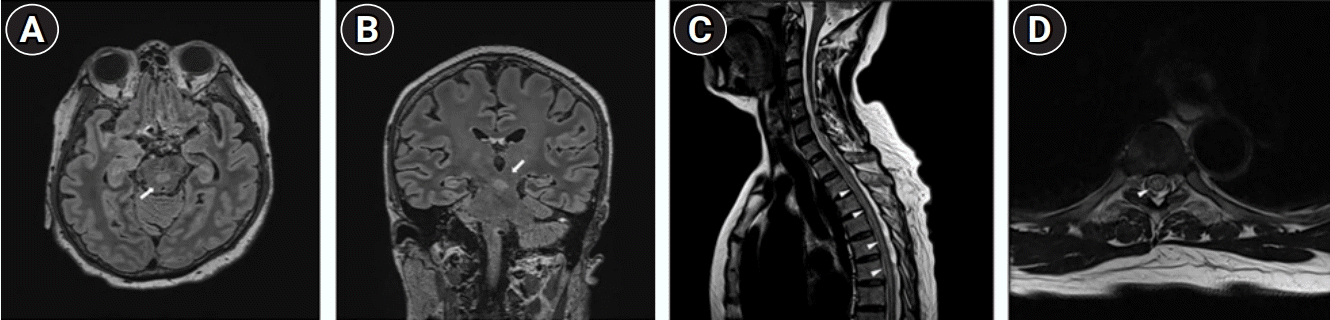
Brain magnetic resonance imaging (MRI) performed on the first day revealed several non-specific small signal changes in the T2-weighted images. Spinal cord MRI showed long segmental signal intensity changes in the spinal cord, from T1 to T11 on the T2-weight images without swelling or abnormal enhancement (Fig. 1A and B). Cerebrospinal fluid revealed a mild pleocytosis (34 cells/μL) and hyperproteinorrachia (0.65 mg/dL; reference range, 8–43 mg/dL), and negative cultures and polymerase chain reaction for viruses. Intravenous (IV) methylprednisolone (MPDS) treatment was started the day before the onset of fever. On the second day, a fever developed, and alertness was decreased. Then, the fever persisted for 2 days despite the administration of propacetamol (1 g every 6 hours) and ibuprofen (400 mg every 6 hours).
A septic screen, including white blood cell count, C-reactive protein (CRP), erythrocyte sedimentation rate, urinary analysis, had no significant findings. CRP levels were mildly elevated (0.99 on day 1, 0.60 on day 2; reference range, ≤0.3 mg/dL), but the white blood cell count was normal. Endocrinologic assays showed unremarkable results. After the administration of oral bromocriptine (2.5 mg/day), the fever was slowly controlled for a few days.
Brain MRI performed on day 3 demonstrated T2-weighted MRI hyperintense lesions involving the midbrain and pons in front of the cerebral aqueduct (Fig. 1C and D). The patient was treated with IV MPDS at 1 g daily for 5 days, but drowsiness and weakness in whole extremities aggravated. We decided to initiate plasma exchange (PLEX) in addition to IV MPDS under the consideration of severe encephalomyelitis due to brain and spinal cord MRI, and decreased consciousness, and the first PLEX was started on the day of fever. Since the fever did not improve well even after high-dose antipyretics, IV MPDS, and 1st PLEX, bromocriptine was administered on the second day of the fever, and the fever gradually recovered. In a cell-based assay, the serum was positive for anti-MOG immunoglobulin G (IgG) antibodies and negative for anti–aquaporin-4 (AQP4) IgG antibodies. Thus, a diagnosis of MOGAD was made according to the core clinical and neuroimaging characteristics and seropositivity for MOG antibody [1,6]. The patient finally underwent ten courses of PLEX.
DISCUSSION
The present case showed that persistent fever in MOGAD could be an uncommon manifestation. AQP4-NMOSD can also be expressed through endocrinopathy, autonomic dysfunction, and dysregulated thermoregulation as a diencephalic syndrome or hypothalamic involvement and has rarely been reported [4,7].
MOGAD has a phenotype similar to AQP4-NMOSD, but a rare and diverse phenotype has been revealed recently [1-3]. The poorly controlled persistent fever, in this case, can also be thought of as a form of diencephalic syndrome in AQP4-NMOSD. At the beginning of hospitalization, the fever did not develop, and no diencephalic lesions were observed on brain MRI. In addition, despite the use of antipyretics, the fever was not fully controlled, and no obvious cause was found in other septic screens or endocrinologic assays. The lesion in the upper brainstem within the follow-up brain MRI was anterior to the periaqueductal gray, a component of the central thermoregulatory pathways from the hypothalamus to the spinal cord, and could be the cause of the patient's persistent fever with unknown origin. However, pleocytosis in the cerebrospinal fluid, increased mild CRP, and possible aspiration due to decreased consciousness can be difficult to completely rule out as causes of fever. Nevertheless, since there were no specific findings on the patient's septic screen, and the fever was relatively well-controlled with bromocriptine, it could be assumed that it is a central fever caused by damage to the thermoregulatory pathway.
In conclusion, we report a rare case of MOGAD presenting with bromocriptine-responsive fever. MOGAD can present as a fever of unknown origin with or without other demyelinating symptoms, and hence, a workup for MOGAD is needed to treat it appropriately.