INTRODUCTION
Nocardiosis is an infectious disease that can be either localized or disseminated. Nocardiosis typically occurs in adults aged 30–50 years, with male predominance, and primarily affects individuals with severe immune dysfunction [1,2]. Because the symptoms of nocardiosis are nonspecific, diagnosis is often challenging and easily missed, with high mortality (>50%) in patients with disseminated or cerebral nocardiosis [1]. The infection is usually confined to the lungs and disseminated or cerebral nocardiosis is relatively rare. Here, we report a rare case of disseminated Nocardia farcinica infection, including cerebral nocardiosis, in an immunosuppressed man, who was successfully managed with a combination of antibiotics.
CASE REPORT
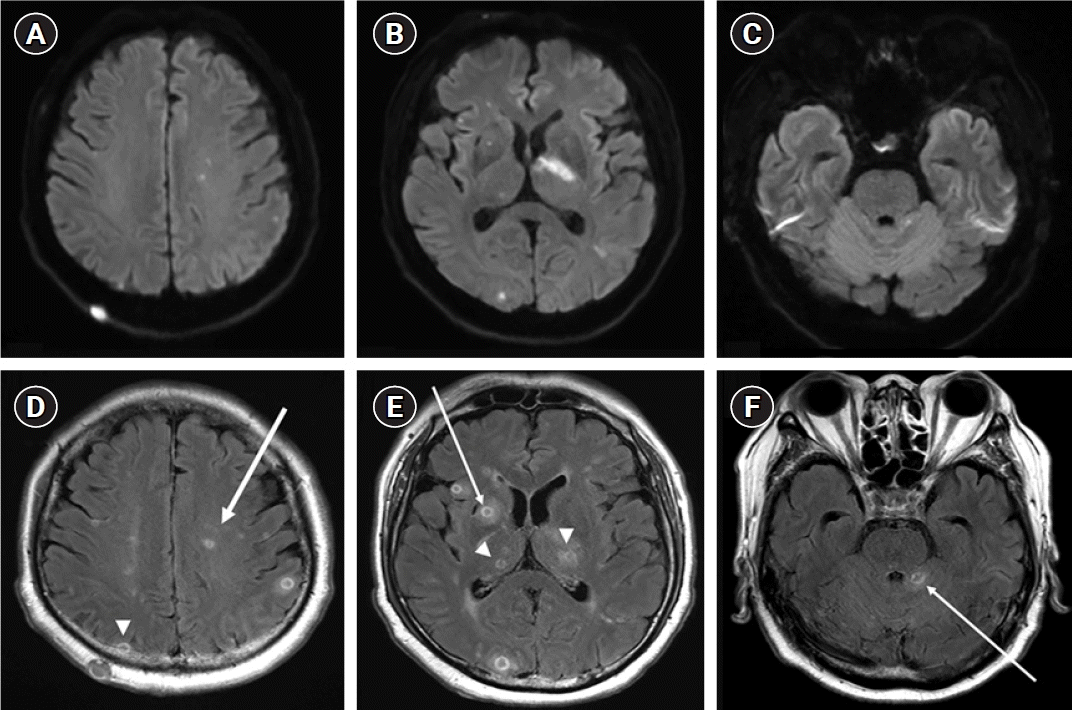
A 71-year-old man presented with sudden-onset motor aphasia, motor weakness of the right limb, and fever (38.3 °C). The patient had a history of hypertension and idiopathic thrombocytopenia purpura (ITP) 5 years prior to admission and was taking 7.5 mg prednisolone per day and 50 mg azathioprine per day for ITP. One month before the onset of the present illness, chest radiography revealed heterogeneous opacities with consolidation in both the lower lobes. A wedge resection was performed to exclude the possibility of lung cancer. Cytomegalovirus pneumonia was confirmed and the patient was treated accordingly. Initial brain magnetic resonance imaging revealed multiple scattered diffusion-restricted lesions, which were present in both hemispheres, thalami, cerebella, and right basal ganglia (Fig. 1A-C). The lesions demonstrated central hypointense signals on T1 weighted imaging with corresponding hyperintense signals on T2 weighted imaging with ring enhancement (Fig. 1D-F).
Initial blood tests showed leukocytosis (19,170/mm3), including 89% polymorphonuclear cells, and elevated C-reactive protein (16.10 mg/dL). A white blood cell count of 580/mm3 (80% neutrophils) and protein level of 152 mg/dL were observed in the initial cerebrospinal fluid test. The brain lesions were thought to be brain abscesses with meningitis and treatment was initiated with ceftriaxone, vancomycin, and metronidazole. One week later, his condition rapidly deteriorated with altered consciousness. Second cerebrospinal fluid analysis confirmed white blood cell count of 684/mm3 and protein level of 107 mg/dL. Abdominopelvic and chest computed tomography revealed multiple cutaneous and peritoneal nodules resulting from hematogenous spread (Supplementary Fig. 1). We made an incision and drained the nodules on the skin. Initially, filamentous acid-fast bacilli (AFB) were not observed in the AFB stains. Moreover, the AdvanSure tuberculosis/nontuberculous mycobacteria real-time PCR kit (AdvanSure; LG Life Science) yielded negative results. However, for suspected nocardiosis, we rechecked the tuberculosis culture media obtained during a previous hospitalization and found growing species in the tuberculosis culture media. Additional testing was required, and filamentous AFB were observed in the modified AFB stains of the specimens (Supplementary Fig. 2). Attempts to identify the organism using matrix-associated laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS, Bruker Daltonics) in the in vitro diagnostic mode yielded no results. However, N. farcinica was identified only in the research use only (RUO) mode. To confirm the results obtained in the RUO mode, we immediately performed full-length 16S ribosomal ribonucleic acid (rRNA) sequencing and obtained basic local alignment search tool (BLAST) results against the National Center for Biotechnology Information 16S rRNA database, which showed the best match to be N. farcinica (1,389/1,389, 100%). We re-evaluated the previously resected lung specimens and confirmed the presence of N. farcinica. As the guardian refused a brain biopsy, stereotactic cerebral biopsy could not be performed. We changed the antibiotics to imipenem and trimethoprim-sulfamethoxazole (TMP/SMX) to treat the disseminated nocardiosis, including cerebral nocardiosis. We identified antimicrobial susceptibility to TMP/SMX. After maintaining the antibiotics for approximately 56 days, the patient recovered full consciousness, and the motor weakness of the right limb improved.
DISCUSSION
Nocardiosis is a rare infection caused by weakly acid-fast gram-positive bacteria belonging to the Mycobacteriaceae family [3]. Although 54 species of Nocardia have been implicated in human infections, N. farcinica is one of the six most common species [4]. Cerebral abscesses caused by N. farcinica have been increasingly diagnosed because of the growing population of immunosuppressed hosts and improvements in diagnostic capabilities [3]. However, cerebral abscesses are relatively rare and account for approximately 2% of all cerebral abscesses [5]. Retroperitoneal abscesses are also rare [6]. To detect Nocardia, the culture identification of Nocardia is traditionally carried out through biochemical tests and phenotypic methods including Gram staining, modified acid-fast staining, and chromatography. However, these methods are limited in terms of species-level identification. Using MALDI-TOF MS, it is possible to rapidly and accurately identify microorganisms in a database. However, for relatively rare microorganisms, the results may not be definitive owing to the possible absence of a reference spectrum in the database. According to previous studies, species-level identification has been reported in only 14.9%–80.4% of Nocardia isolates [7,8]. Therefore, we identified Nocardia, a relatively rare microorganism, using 16S rRNA sequencing in conjunction with MALDI-TOF MS. Although requiring more time and cost, 16S rRNA sequencing and BLAST analysis can provide additional information for the identification of detailed differences between species. A previous study showed that stereotactic cerebral biopsy should be considered early in the diagnostic workup of immunocompromised patients owing to the aggressive course of cerebral nocardiosis [9]. However, because the patient’s guardian refused a cerebral biopsy, stereotactic cerebral biopsy could not be performed. N. farcinica is usually resistant to multiple antimicrobial agents, particularly broad-spectrum cephalosporins, which makes treatment difficult [10]. TMP/SMX is the most effective treatment for central nervous system infections caused by Nocardia [10]. Numerous agents, including imipenem, are considered acceptable second-line treatments. Combination therapy should be considered for patients with severe disseminated central nervous system infections. Prognosis is related to the severity of the underlying disease, site of infection, patient immune function, and presence of drug resistance. The present study had several limitations. First, this was a single-patient single-center study. Additionally, no microorganisms were detected in the brain. Another important limitation is the lack of understanding of the molecular and cellular mechanisms that lead to disseminated infection.
As the use of immunosuppressants increases, the accurate identification of N. farcinica is important.