INTRODUCTION
Bacterial meningitis is a life-threatening disease with high mortality and morbidity [1]. Of the potential etiologies of bacterial meningitis, Streptococcus agalactiae is most common in neonates and rare in adults [2]. In adult patients (older than 16 years) subarachnoid hemorrhage (SAH) was diagnosed in <1% of cases in the setting of bacterial meningitis [3]. Unfortunately, SAH in patients with bacterial meningitis is associated with high case fatality and morbidity, with significant complications, including impaired level of consciousness, respiratory failure, circulatory shock, and seizures [3]. Well known risk factors for Group B Streptococcus (GBS) meningitis include previous occurrence, older age (>65 years), diatetes mellitus, heart disease, obesity, immunodeficiencies, and cancer. In one study, patients with GBS meningitis and concurrent SAH were over three times more likely to die (54% vs. 16%) than patients without SAH [3]. The mechanisms behind the development of SAH secondary to bacterial meningitis are not fully understood but leading theories suggest the inflammatory milieu of the vascular system can lead to breakdown of blood vessels. There have been associations identified between the presence of saccular or mycotic aneurysms and concurrent bacterial meningitis with SAH [3]. There is suspicion that the aneurysms are a risk factor for the development of both bacterial meningitis and the potential sequelae of SAH [4]. Deliran et al. [3] have produced a nation-wide cohort study in the Netherlands with a sample of 22 patients experiencing concurrent SAH with GBS. Notably, their study found that while only one third of these patients were diagnosed with SAH on admission, none of the patients presented with headache. In addition, the neuroimaging associated with each patient, when available, was unable to significantly correlate to the symptoms each patient presented with. Mook-Kanamori et al. [5] found the increased risk of SAH secondary to any bacterial meningitis increased five-fold when patients were on anticoagulants. Both Deliran et al. [3] and Mook-Kanamori et al. [5] were unable to identify any significantly different clinical presentations when patients had both SAH and GBS meningitis. This is the first published case of GBS meningitis identified following classic SAH presentation.
CASE REPORT
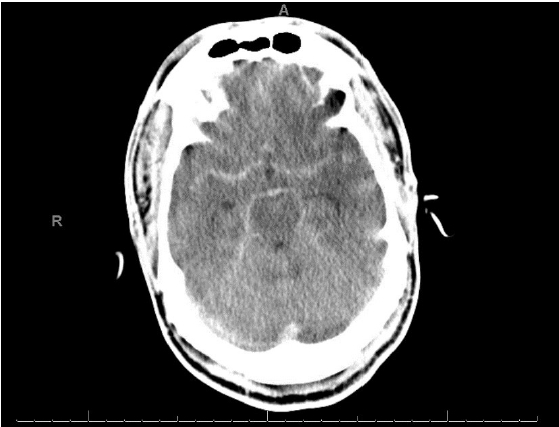
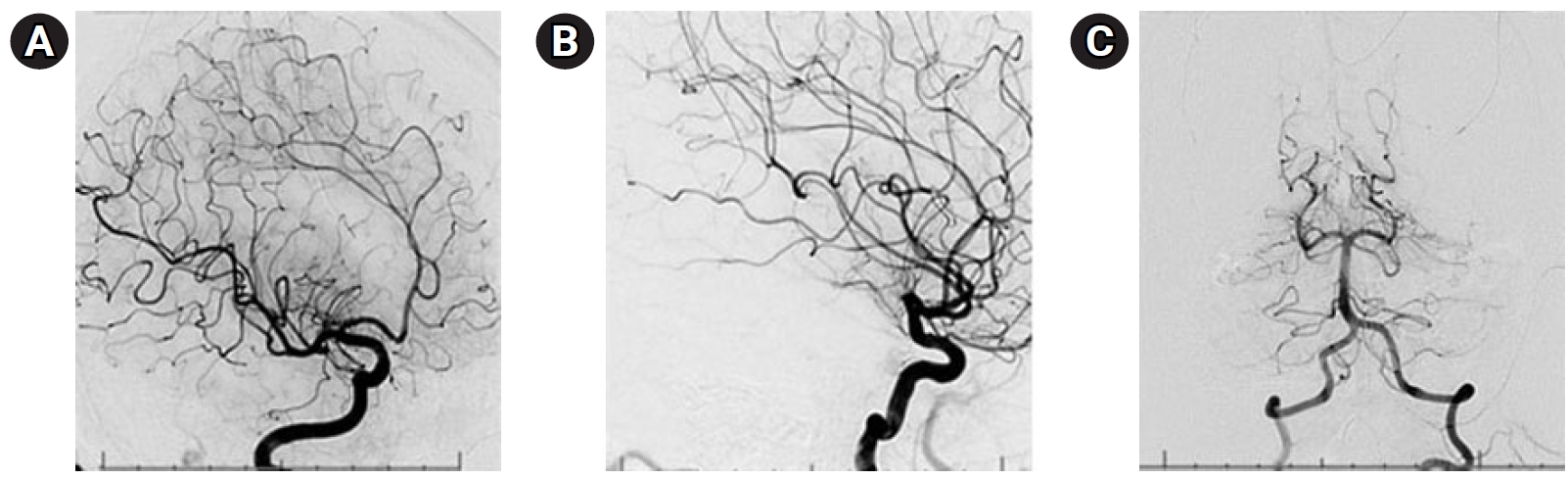
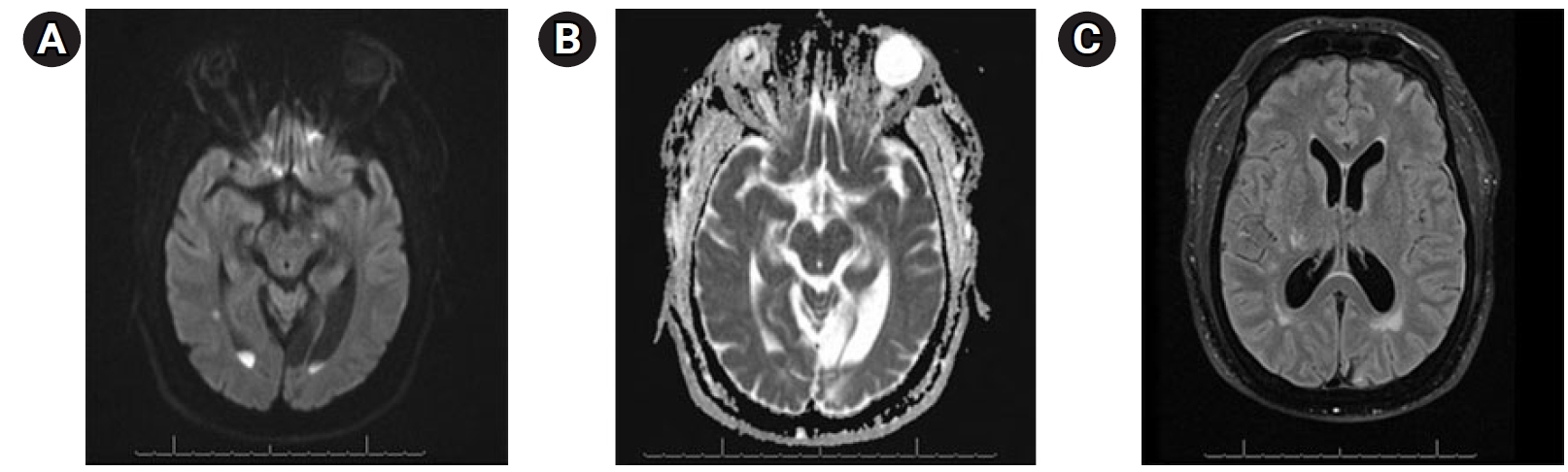
A 58-year-old man presented with a 5-day history of abdominal pain, myalgias, arthralgias, fever, diffuse headache, nausea, vomiting, diarrhea, and poor oral intake. The patient had a medical history of end-stage renal disease requiring hemodialysis, coronary artery disease status post coronary artery bypass grafting and multiple stents (most recently 3 weeks prior), previous stroke with residual blindness bilaterally, diabetes mellitus, and hypertension While in the emergency department (ED), he suddenly developed “the worst headache of his life,” followed by a 4-minute tonic-clonic convulsion. His presentation was notable for somnolence without focal neurological deficits, consistent with Hunt Hess grade IV SAH. He was subsequently intubated, and head computed tomography (CT) demonstrated findings consistent with SAH (Fig. 1). Neurosurgery was consulted and he was promptly transported for urgent diagnostic cerebral angiography. Digital subtraction angiography (DSA) was performed and was notable for a normal cerebral angiogram without any evidence of mycotic or saccular aneurysms, dural arteriovenous fistulas, or other etiologies for the patient’s SAH (Fig. 2). The patient was subsequently admitted to the neurocritical care unit, and urine culture was positive for GBS bacteriuria. Lumbar puncture was performed on hospital day 2 (HD 2), and results were suggestive of bacterial meningitis (Table 1). The electroencephalogram was abnormal, with a slow and low amplitude background, but no seizures. Magnetic resonance imaging performed on HD 2 provided evidence of scattered areas of increased fluid-attenuated inversion recovery signal with associated enhancement and foci of restriction diffusion in the bilateral cerebral sulci, sylvian fissures and fourth ventricular outflow tract, suggestive of diffuse meningitis with associated encephalitis (Fig. 3). There was additional suspected pus material with restriction effusion layering at the posterior horns of the lateral ventricles. A repeat head CT was performed for slight decline in mental status with imaging negative for worsening bleed and overall was stable with slightly worsening edema. Urine culture was positive for GBS, and the patient continued to have intermittent fevers. Lumbar puncture was repeated on HD 6 and cerebrospinal fluid (CSF) samples continued to not grow any colonies on our in-house laboratory testing. A CSF sample was then sent to Mayo Clinic for further culturing and susceptibility analysis. Their laboratory was able to determine the presence of S. agalactiae in the patient’s CSF, providing culture-proven evidence of GBS meningitis. The patient was continued on antibiotics and on HD 7 the patient's neurological exam was significantly improved with eyes opening spontaneously, and the patient beginning to follow commands. He was successfully extubated the following day. His transthoracic and transesophageal echocardiograms were negative for the presence of vegetations, and he continued clinical improvement until his hospital discharge on HD 15. As S. agalactiae was detected in the CSF by polymerase chain reaction (PCR), our broad workup for potential bacteremia was negative, making bacteremia a less likely diagnosis.
On admission, our patient was started on vancomycin and ampicillin for 7 days, amphotericin B and metronidazole for 3 days, acyclovir for 4 days, and cefepime for 5 days. The final antibiotic plan was to continue ceftriaxone to fulfill a 4-week period of treatment. At the time of discharge, we transitioned his antibiotic regimen to cefazolin with dialysis, to avoid the need for a peripherally-inserted central catheter.
Patient was discharged home HD 15 and received follow-up with neurology 8 months later. The patient was then referred for an outpatient sleep study, with no concerns at that time. The patient is currently doing well and was last seen on December 2, 2022, following up with sleep medicine.
DISCUSSION
Bacterial meningitis in adults is rare, and confers significant morbidity and mortality. Its clinical presentation has been generally well-characterized in the literature. The question of utility emerges when presented with a classical presentation of another condition and the need to reject confirmation bias to explore other possible differentials. Our patient’s presentation would most commonly be associated with SAH, that would typically follow with confirmation by DSA and microorganisms seen with CSF samples that appear to be cloudy would have been expected.
In the case we present, our patient described “the worst headache of his life” and had CT findings consistent with SAH. DSA found no obvious source of bleeding and subsequently another differential diagnosis became more likely; meningoencephalitis. No bacteria were identified under microscopy despite repeated attempts with new samples. Fortunately, we were able to contact the Mayo Clinic and through their BioFire Panel PCR, determined the presence of S. agalactiae. When our CSF samples were unremarkable for bacteria, we did not pursue culture and antibiotic susceptibility testing. Without having confirmatory PCR evidence, the patient initially continued to receive empiric broad spectrum antibiotic treatment.
There are few case studies exploring the care of patients with S. agalactiae bacterial meningitis, and even fewer collections of reported atypical presentations. As such, we felt it was important to illustrate and reiterate the key principles of care when presented with such an uncommon clinical constellation. Improving awareness of this presentation of GBS meningitis, in combination with the negative in-house CSF samples, calls to attention the value of treating empirically based on clinical suspicion. Furthermore, this raises awareness of the importance of correctly evaluating the resources available for laboratory testing.
With such significant morbidity and mortality associated with this specific presentation of bacterial meningitis, the benefits of earlier diagnosis and treatment are supported in this case report and can contribute to the improvements in outcomes for such patients [2,3]. These clinical interventions can be best practiced, and are more readily apparent, to better informed clinicians.