Samuel, Nguyen, and Choi: Pharmacologic Characteristics of Corticosteroids
Cited By
Citations to this article as recorded by

Exploring the role of antibiotics and steroids in managing respiratory diseases
Dinesh K. Chellappan, Parteek Prasher, Shakti D. Shukla, Tong W. Yee, Tiong K. Kah, Toh W. Xyan, Tang W. Kid, Teoh H. Si, Ting S. Weng, Nagashekhara Molugulu, Lakshmana P. Sakthivel, Jestin Chellian, Thiagarajan Madheswaran, Himaja Malipeddi, Yogendra Sin
Journal of Biochemical and Molecular Toxicology.2022;[Epub]
CrossRef A multicenter real‐life study to determine the efficacy of corticosteroids and olfactory training in improving persistent COVID‐19‐related olfactory dysfunction
Alfonso Luca Pendolino, Giancarlo Ottaviano, Juman Nijim, Bruno Scarpa, Giulia De Lucia, Cecilia Berro, Piero Nicolai, Peter J. Andrews
Laryngoscope Investigative Otolaryngology.2023; 8(1): 46.
CrossRef Application of microbial 3-ketosteroid Δ1-dehydrogenases in biotechnology
Ali Rohman, Bauke W. Dijkstra
Biotechnology Advances.2021; 49: 107751.
CrossRef A critical evaluation of glucocorticoids in the management of severe COVID-19
Cinzia Solinas, Laura Perra, Marco Aiello, Edoardo Migliori, Nicola Petrosillo
Cytokine & Growth Factor Reviews.2020; 54: 8.
CrossRef The role of natural products from medicinal plants against COVID-19: traditional medicine practice in Tanzania
Stephano Hanolo Mlozi
Comparison of the effect of intravenous dexamethasone and methylprednisolone on the treatment of hospitalized patients with COVID-19: a randomized clinical trial
Zahra Habibi Dastenae, Azadeh Bahadori, Marziyeh Dehghani, Majid Asadi-Samani, Iman Izadi, Hadi Raeisi Shahraki
International Journal of Infectious Diseases.2022; 122: 659.
CrossRef Hyaluronic acid-conjugated liposomes loaded with dexamethasone: A promising approach for the treatment of inflammatory diseases
Kamila Bohne Japiassu, Francois Fay, Alessandro Marengo, Sebastião A. Mendanha, Catherine Cailleau, Younès Louaguenouni, Qinglin Wang, Stéphanie Denis, Nicolas Tsapis, Thais Leite Nascimento, Eliana Martins Lima, Elias Fattal
International Journal of Pharmaceutics.2023; 639: 122946.
CrossRef Practical Guidance for Prevention and Management of Glucocorticoid-Induced Osteoporosis for the Allergist/Immunologist
Natalia Weare-Regales, Stephanie N. Hudey, Richard F. Lockey
The Journal of Allergy and Clinical Immunology: In Practice.2021; 9(5): 1841.
CrossRef Emerging nanoparticle platforms to improve the administration of glucocorticoids
Barbara Tessier, Nicolas Tsapis, Elias Fattal, Laurence Moine
Journal of Controlled Release.2023; 358: 273.
CrossRef DFT calculations, spectroscopic investigations, and molecular docking study of Methylprednisolone with some selective cancer proteins
Jyotshna Saikia, Th. Gomti Devi, T. Karlo
Materials Today: Proceedings.2023;[Epub]
CrossRef Focused mutagenesis in non-catalytic cavity for improving catalytic efficiency of 3-ketosteroid-Δ1-dehydrogenase
Yajiao Zhang, Minjie Liu, Huijing Wang, Juan Lin, Fener Chen
Molecular Catalysis.2022; 531: 112661.
CrossRef Short-term exposure to dexamethasone at environmentally relevant concentrations impairs embryonic development in Cyprinus carpio: Bioconcentration and alteration of oxidative stress-related gene expression patterns
Veronica Margarita Gutiérrez-Noya, Leobardo Manuel Gómez-Oliván, Idalia Casas-Hinojosa, Sandra García-Medina, Karina Elisa Rosales-Pérez, José Manuel Orozco-Hernández, Gustavo Axel Elizalde-Velázquez, Marcela Galar-Martínez, Octavio Dublán-García, Hariz I
Science of The Total Environment.2023; 898: 165528.
CrossRef Hydrocortisone may spare head growth, but the debate for steroid use rages on
Hellen Ko, Ashwini Lakshmanan, Jessie R. Maxwell
Pediatric Research.2023; 94(6): 1867.
CrossRef Expeditious synthesis and preliminary antimicrobial activity of deflazacort and its precursors
Anna Esposito, Eliana De Gregorio, Maria De Fenza, Daniele D'Alonzo, Anil Satawani, Annalisa Guaragna
RSC Advances.2019; 9(37): 21519.
CrossRef
In silico
validation of hyaluronic acid – drug conjugates based targeted drug delivery for the treatment of COVID-19
Mohan Mani, Mahesh Vellusamy, Thirumalaisamy Rathinavel, Pullar Vadivel, Manuel Dauchez, Riaz Khan, Vincent Aroulmoji
Journal of Biomolecular Structure and Dynamics.2024; : 1.
CrossRef Alternatives to Hydrocortisone for Hemodynamic Support in Septic Shock Management Due to Medication Shortage
Mohammed Aldhaeefi, Abdulrahman Alshaya, Sanaa Belrhiti, Dhakrit Rungkitwattanakul
Critical Care Explorations.2023; 5(7): e0940.
CrossRef Hyponatremia in Patients With Multisystem Inflammatory Syndrome in Children
Tatyana Mills, Aditi Trivedi, Adriana H. Tremoulet, Daniel Hershey, Jane C. Burns
Pediatric Infectious Disease Journal.2021; 40(9): e344.
CrossRef Fasting with adrenal insufficiency: Practical guidance for healthcare professionals managing patients on steroids during Ramadan
Sufyan Hussain, Shazia Hussain, Ruzwan Mohammed, Karim Meeran, Nazim Ghouri
Clinical Endocrinology.2020; 93(2): 87.
CrossRef Genotyping, drug resistance and virulence factors of Candida species isolated from patients using long‐term inhaled steroids
Nursel Dikmen, Nizami Duran, Emrah Ay, Funda Cimen, Erhan Tek
International Journal of Clinical Practice.2021;[Epub]
CrossRef Steroid Use for Recovery of advanced atrioVentricular block Immediately after VALvular surgery (SURVIVAL): A preliminary randomized clinical trial
Saeed Ghodsi, Farzad Masoudkabir, Zahra Hosseini, Tahereh Davarpasand, Negin Yavari, Mehrnaz Mohebi, Azita H. Talasaz, Arash Jalali, Seyed H. Ahmadi‐Tafti, Jamshid Bagheri, Hakimeh Hasanzadeh
Journal of Cardiovascular Electrophysiology.2022; 33(4): 575.
CrossRef A comparison of intravenous methylprednisolone and hydrocortisone for the treatment of acute inflammatory bowel disease
Cameron Schauer, Victoria Avery, Sam Seleq, Paras Garg, Michael T M Wang, Michael Chieng, Charlotte Rowan, Anurag Sekra, Mark Lane, Russell Walmsley
Journal of Gastroenterology and Hepatology.2021; 36(10): 2762.
CrossRef Effect of glucocorticoids on the development of COVID‐19‐associated pulmonary aspergillosis: A meta‐analysis of 21 studies and 5174 patients
Zia Hashim, Alok Nath, Ajmal Khan, Mansi Gupta, Anup Kumar, Riksoam Chatterjee, Radha Krishan Dhiman, Martin Hoenigl, Naresh Kumar Tripathy
Dexamethasone: Therapeutic Applications, Targets and Translation
Rishabh S. Hirday, Grace H. Tam, Audrey A. O’Neill, Mollie S. Davis, Rene S. Schloss
Efficacy of Preemptive Dexamethasone versus Methylprednisolone in the Management of Postoperative Discomfort and Pain after Mandibular Third Molar Surgery: A Systematic Review and Meta-Analysis
Anupam Singh, Kalyana Chakravarthy Pentapati, Murali Venkata Rama Mohan Kodali, Komal Smriti, Vathsala Patil, Gandham Lekha Chowdhary, Srikanth Gadicherla, Ho Soonmin
The Scientific World Journal.2023; 2023: 1.
CrossRef THE ACTION OF COX/LOX INHIBITORS ON ANTIOXIDANT SYSTEM AND MORPHOLOGICAL STATE OF RAT’S COLON MUCOSA UNDER THE CONDITIONS OF STRESS
N. V. Denysenko, O. Ya. Sklyarov
Medical and Clinical Chemistry.2019; (2): 5.
CrossRef Steroid treatment as anti-inflammatory and neuroprotective agent following out-of-hospital cardiac arrest: a randomized clinical trial
Laust Emil Roelsgaard Obling, Rasmus Paulin Beske, Sebastian Wiberg, Fredrik Folke, Jacob Eifer Moeller, Jesper Kjaergaard, Christian Hassager
Management of patients on systemic steroids: An oral surgery perspective
Vikash Patel, Shrina Nathwani, Naomi Rahman
Dental Update.2022; 49(9): 749.
CrossRef Changes of L-Arginine Metabolism in Rat`S Colon Mucosa Under the Conditions of COX/LOX Inhibition and Acute Stress Action
Nataliya Denysenko, Alexander Sklyarov
Biosciences Biotechnology Research Asia.2021; 18(2): 313.
CrossRef Guía de práctica clínica para el manejo de la neumonía adquirida en la comunidad
Jorge Alberto Cortés, Sonia Isabel Cuervo-Maldonado, Laura Cristina Nocua-Báez, Martha Carolina Valderrama, Edgar Alberto Sánchez, Alfredo Saavedra, July Vianneth Torres, Diana Paola Forero, Carlos Arturo Álvarez, Aura Lucía Leal, Jairo Enrique
Revista de la Facultad de Medicina.2021; 70(2): e93814.
CrossRef Coronavirus Disease 2019 and Hypertension: How Anti-hypertensive Drugs Affect COVID-19 Medications and Vice Versa
Aida Doostkam, Alireza Hosseinpour, Kamyar Iravani, Leila Malekmakan, Abdolreza Haghpanah, Fatemeh Masjedi, Zeinab Karimi, Hossein Rouzbeh, Jamshid Roozbeh
Current Drug Safety.2023; 18(2): 125.
CrossRef The The use of corticosteroid therapy for COVID-19 patients: an evidence-based overview
Ricardo Guimarães Amaral, Ricardo Ruan Rocha Santana, Bárbara Oliva Barbosa, Yuri Barbosa Araújo, Sandra Lauton Santos, Luciana Nalone Andrade
REVISTA CIÊNCIAS EM SAÚDE.2022; 12(3): 4.
CrossRef The impact of single dose intravenous dexamethasone as an adjunctive therapy for primary treatment on concentrations of inflammatory biomarkers in children with Kawasaki disease
Jung Eun Kwon
Pediatric Emergency Medicine Journal.2022; 9(1): 23.
CrossRef Neuroinflammation Treatment via Targeted Delivery of Nanoparticles
Susana R. Cerqueira, Nagi G. Ayad, Jae K. Lee
Frontiers in Cellular Neuroscience.2020;[Epub]
CrossRef Psycho-Behavioural Changes in Dogs Treated with Corticosteroids: A Clinical Behaviour Perspective
Lorella Notari, Roxane Kirton, Daniel S. Mills
Effects of Early Initiation of High-Dose Dexamethasone Therapy on Pro-Inflammatory Cytokines and Mortality in LPS-Challenged Mice
Ji-young Son, Won Gun Kwack, Eun Kyoung Chung, Sooyoung Shin, Yeo Jin Choi
Biotransformation of Δ1-Progesterone Using Selected Entomopathogenic Filamentous Fungi and Prediction of Its Products’ Bioactivity
Anna Panek, Patrycja Wójcik, Alina Świzdor, Maciej Szaleniec, Tomasz Janeczko
International Journal of Molecular Sciences.2023; 25(1): 508.
CrossRef 11β-Hydroxysteroid Dehydrogenase Type 1 as a Potential Treatment Target in Cardiovascular Diseases
Daria Kupczyk, Renata Studzińska, Renata Kołodziejska, Szymon Baumgart, Martyna Modrzejewska, Alina Woźniak
Journal of Clinical Medicine.2022; 11(20): 6190.
CrossRef A New 3-Ketosteroid-Δ1–Dehydrogenase with High Activity and Broad Substrate Scope for Efficient Transformation of Hydrocortisone at High Substrate Concentration
Yu Wang, Rui Zhang, Jinhui Feng, Qiaqing Wu, Dunming Zhu, Yanhe Ma
Microorganisms.2022; 10(3): 508.
CrossRef Utilizing Protein–Peptide Hybrid Microarray for Time-Resolved Diagnosis and Prognosis of COVID-19
Peiyan Zheng, Baolin Liao, Jiao Yang, Hu Cheng, Zhangkai J. Cheng, Huimin Huang, Wenting Luo, Yiyue Sun, Qiang Zhu, Yi Deng, Lan Yang, Yuxi Zhou, Wenya Wu, Shanhui Wu, Weiping Cai, Yueping Li, Xiaoneng Mo, Xinghua Tan, Linghua Li, Hongwei Ma, Baoqing Sun
Microorganisms.2023; 11(10): 2436.
CrossRef Reconstruction of the Steroid 1(2)-Dehydrogenation System from Nocardioides simplex VKM Ac-2033D in Mycolicibacterium Hosts
Svetlana R. Fufaeva, Dmitry V. Dovbnya, Tanya V. Ivashina, Andrei A. Shutov, Marina V. Donova
Microorganisms.2023; 11(11): 2720.
CrossRef Δ1-Dehydrogenation and C20 Reduction of Cortisone and Hydrocortisone Catalyzed by Rhodococcus Strains
Stefania Costa, Federico Zappaterra, Daniela Summa, Bruno Semeraro, Giancarlo Fantin
Supercritical Antisolvent Precipitation of Corticosteroids/β-Cyclodextrin Inclusion Complexes
Stefania Mottola, Iolanda De Marco
Antioxidant Associated Antihypertensive Performance of Purified Gambir (Uncaria gambir Roxb.) on Prednisone Salt-Induced Hypertensive Rats
Dita Permatasari, Puti Khairunnisa, Kania Rosyari, Fatma Sri Wahyuni, Yufri Aldi, Armenia Armenia
Open Access Macedonian Journal of Medical Sciences.2022; 10(A): 390.
CrossRef Steroid Allergy in a Patient With Behçet's Disease
Bailey M. Harrison, R. John Looney, Zoё R. Williams, David A. DiLoreto
Ophthalmic Surgery, Lasers and Imaging Retina.2021; 52(8): 447.
CrossRef Quantitative Spectrophotometric Estimation of Prednisolone in Tablet Dosage Form Using Eco-friendly Green Solvent and by applying Beer-Lambert’s Law Mathematical Equation Method
Sana Tabassum, Ajitha M
Asian Journal of Research in Chemistry.2021; : 155.
CrossRef ANTI-INFLAMMATORY EFFECTS OF Vitex trifolia LEAVES HYDROALCOHOLIC EXTRACT AGAINST HYDROGEN PEROXIDE (H2O2)- AND LIPOPOLYSACCHARIDE (LPS)-INDUCED RAW 264.7 CELLS
AHMAD TAMIM GHAFARI, AISYAH HASYILA JAHIDIN, YUSLINA ZAKARIA, MIZATON HAZIZUL HASAN
Malaysian Applied Biology.2022; 51(4): 185.
CrossRef UV-VIS AND FTIR SPECTROSCOPIC PROFILE OF GAMETOPHYTE AND SPOROPHYTE ETHANOLIC EXTRACT OF ANEMIA SCHIMPERIANA C. PRESL SUBSP. WIGHTIANA (GARDNER) FRASER-JENK. AND CYATHEA GIGANTEA (WALL. EX. HOOK.) HOLTT.
SILVIA JULIET IRUTHAYAMANI, M. JOHNSON
Romanian Journal of Biophysics.2023; 33(4): 183.
CrossRef Mineralocorticoid Deficiency as an Early Presenting Symptom of Allgrove Syndrome With Novel Mutation: A Case Report
Hashem A AlOmran , Fadi Busaleh, Zahra Alhashim , Manal AlHelal, Yasen Alsaleh, Aida AlJabri, Zahra A AlGhadeer , Fatimah Y AlHejji, Mousa AlMazeedi, Abdulelah M Al dandan
Abstract
Corticosteroids (CSs) are used frequently in the neurocritical care unit mainly for their antiinflammatory and immunosuppressive effects. Despite their broad use, limited evidence exists for their efficacy in diseases confronted in the neurocritical care setting. There are considerable safety concerns associated with administering these drugs and should be limited to specific conditions in which their benefits outweigh the risks. The application of CSs in neurologic diseases, range from traumatic head and spinal cord injuries to central nervous system infections. Based on animal studies, it is speculated that the benefit of CSs therapy in brain and spinal cord, include neuroprotection from free radicals, specifically when given at a higher supraphysiologic doses. Regardless of these advantages and promising results in animal studies, clinical trials have failed to show a significant benefit of CSs administration on neurologic outcomes or mortality in patients with head and acute spinal injuries. This article reviews various chemical structures between natural and synthetic steroids, discuss its pharmacokinetic and pharmacodynamic profiles, and describe their use in clinical practice.
Key Words: Corticosteriods; Glucocorticoids; Inflammation; Immunosuppressive
INTRODUCTION
Corticosteroids (CSs) are a class of steroid hormones that are produced and secreted by the adrenal glands in response to pituitary adrenocroticotropic hormone, and regulated by hypothalamic croticotropin releasing hormone. These hormones are responsible for regulating major endocrine system functions, including managing stress and controlling homeostasis. The main CSs produced by the adrenal cortex are cortisol (glucocorticoids) and aldosterone (mineralocorticoids). Aldosterone influences sodium and water balance, while cortisol exerts its effect by preventing the release of inflammation mediators [
1]. In the late 1940s, high blood cortisol levels were discovered in cushingoid patients and its anti-inflammatory effects were first demonstrated in patients with rheumatoid arthritis [
2]. Since then, numerous investigators have established the ability of physiologic levels of these hormones to alter inflammation and immune functions [
3-
6]. Currently, CSs find use in the treatment of various neurological diseases, inflammation, pain, autoimmune disorders, and cancer. The purpose of this article is to briefly review the various chemical structural differences between natural and synthetic steroids, discuss their pharmacokinetic and pharmacodynamic profiles, and describe their use in neurocritical care clinical practice.
PHARMACOKINETICS
Cortisol is present within the plasma in three different forms; free cortisol, protein bound, and cortisol metabolite. About 5% of circulating cortisol is unbound and, roughly, 80% of the circulating cortisol is bound to cortisol binding globulin (CBG) or albumin. During inflammation, the binding affinity of cortisol declines, rendering a higher free cortisol concentration at the site of interest to alleviate the active inflammatory process. Most synthetic CSs are also found bound to CBG, however, these glucocorticoids analogues bind less efficiently compared to native cortisol. Cortisol metabolites are biologically inactive and bind weakly to circulating plasma proteins [
7-
9].
In the early 1950s, chemical modifications of natural steroids revealed a number of structural features essential for their biological activities. Cortisol includes a cyclopen-tenoperhydrophenanthrene nucleus, made up of three 6-carbon rings and a single 5-carbon pentane ring. Cortisol has 21 carbon atoms with a 2-carbon side chain attached to position 17 and methyl groups at C-10 and C-13 [
10]. Chemical alterations at various positions of the steroid molecule lead to synthetic analogous of cortisol with increased glucocorticoid and/or mineralocorticoid activities [
11,
12]. For example, cortisone is derived from cortisol by substituting the hydroxyl group at C-11 with a carbonyl group, whereas prednisolone results from the addition of a double bond between the C-1 and C-2 positions of cortisol; methylation of prednisolone at C-6 produces methylprednisolone (
Fig. 1) [
10]. Glucocorticoids that are fluorinated at the 9-alpha position include dexamethasone, fludrocortisone and betamethasone [
10]. Both cortisone and prednisolone must be converted to the active metabolites cortisol and prednisone, respectively, prior exerting their action [
13]. A slight change in the molecular structure introduces a variety of synthetic glucocorticoids with diverse potency, half-lives, and mineralocorticoid activities. Some are far more potent than cortisol, and their different pharmacodynamic and pharmacokinetic activities gave them unique characteristic.
Table 1 shows common orally available corticosteroids and compares their potency and mineralocorticoids activity. Notably, fludrocortisone acetate is classified as a mineralocorticoid as it has minimal glucocorticoid activity. It is included in the table to provide perspective on mineralocorticoid potency [
1,
14-
18].
PHARMACODYNAMIC OF CORTICOSTEROIDS
Corticosteroids exert their anti-inflammatory and immunosuppressive effects by interrupting multiple steps in the up-regulation of the immune system. Their suppressive activity is predominantly restricted to cell-mediated immunity [
19]. It is believed that CS inhibit antigen presentation, cytokine production and lymphocytes proliferation by binding to glucocorticoid receptors found throughout the body [
19]. In response to CS administration, lymphocytopenia is induced as a result of redistribution of circulating lymphocytes into other lymphoid compartments. A single dose of CS produces lymphocytopenia within 4 hours of administration which normalizes through redistribution into the circulation away from periphery, rather than destruction, within 24 to 48 hours. Similarly, monocytes and eosinophils, which normally accumulate at the inflammatory site, decrease upon administration of CSs. In contrast, CSs induce neutrophilia via the release of neutrophils from the bone marrow into the circulation, reduction in the migration of neutrophils out of the circulation, and demargination of neutrophils from the vascular lining [
19,
20].
The anti-inflammatory process mediated by CSs is multi-modal and begins with synthesis of lipocortin-1, which then suppresses phospholipase A2, thereby blocking eicosanoid production, and further inhibiting various leukocyte inflammatory events. The end result of this process is inhibition of prostaglandin synthesis and cyclooxygenase (COX-1 and COX-2), thus potentiating the anti-inflammatory effect [
19,
20].
USE OF CORTICOSTEROIDS IN CLINICAL PRACTICE
Because of their myriad effects on the immune system, the clinical utility of CSs is vast, but in clinical practice they are generally used for their anti-inflammatory and immunosuppressive effects. Side effects of these drugs are greater with increased duration of use and higher, supraphysiological doses, thus their use should be limited to specific conditions with careful assessment of the risk versus benefit [
5,
6]. The dose and duration of therapy varies based on the indication. For example, in acute conditions such as multiple sclerosis relapse, a higher dose but shorter course of therapy may be warranted versus chronic conditions such as rheumatoid arthritis where lower maintenance doses are advocated [
21,
22]. The application of CSs in neurologic diseases, though not limited to neuroimmunologic disorders, range from traumatic head and spinal cord injuries to central nervous system infections. The proposed benefit of CSs therapy in brain and spinal cord conditions, include neuroprotection from free radicals, reduced intracranial pressure via decrease in cerebrospinal fluid pressure, and maintenance of normal microvasculature dependability [
22]. Despite these advantages and inspiring results in laboratory studies, clinical trials have been unsuccessful in showing a significant benefit of CSs administration on neurologic outcomes or mortality in patients with head injuries. The largest scale investigation published to date, the Corticosteroid Randomization after Significant Head Injury (CRASH) study, involved over 10,000 patients and was intended to determine the effects of short-term CS infusion on death and disability following significant head injury. The study revealed that the risk of death from all causes, within two weeks of severe head trauma, was higher in the CS group than the placebo group [
23]. Thus, the current guidelines from the Brain Trauma Foundation do not indorse the use of CSs for improving outcome or reducing intracranial pressure in patients with severe head injury [
24].
Methylprednisolone (MP) sodium succinate had been used in spinal trauma patients with the aim of mitigating inflammation, lipid peroxidation, and excitotoxicity associated with the acute injury [
25]. At high doses, it functions as a free radical-scavenger and neuroprotectant, secondary to glucocorticoid receptor-mediated inhibition of phospholipase A2. When given in high doses, CSs impair cytokine generation and enter cell membranes. This alters the physicochemical properties as well as the activities of membrane associated proteins. These effects are deduced from administration of CSs in high doses to effectively treat acute exacerbations [
21,
26].
Multiple prospective and retrospective studies had attempted to evaluate the benefit of MP in patients who suffered acute spine injury. A study that is well known to many and which may have misled many clinicians in managing spine injury patients, was the National Acute Spinal Cord Injury (NASCI I, II, and III) multicentered double blinded randomized studies, that compared MP to placebo/control in acute spinal cord injury patients [
27-
29]. In the first study (NASCI 1), 330 patients were followed for 6 months. Each arm received either 100 mg bolus, then 25 mg every 6 hours for 10 days or 1,000 mg bolus, then 250 mg every 6 hours for 10 days. No significant differences in neurologic outcome between the two groups were seen. However, a statistically significant increase in wound infections was reported in the group who received a high dose [
27]. Authors argued that the reason for lack of differences in the primary outcome was due to the low dose of MP used, which failed to reveal a potential beneficial effect. In NASCI II trail, 487 patients were included and were followed for 1 year. Subjects were randomized into 3 groups, the MP group received MP as a 30 mg/kg bolus, then 5.4 mg/kg/hour for 23 hours, naloxone and placebo were administered for the remaining two arms of the study. The outcome of this study was criticized due to inappropriate reporting of results and statistical analysis. Overall, the neurologic outcome was a negative result, but a post-hoc analysis reported positive results but failed to show any clinical significance. Wound infection and pulmonary embolus were twice as frequent in the treatment group vs. the naloxone and placebo groups [
28]. The third NASCI trial, once again failed to show the benefit of MP in acute spinal cord injury patients. Similar to the NASCIS 2 study, there was no difference between MP and placebo in the outcome of spinal cord injury. The post-hoc analysis identified a statistically significant improvement, but as in the NASCIS 2 results, the clinical significance of this improvement was also questioned. Incidence of severe pneumonia and severe sepsis was higher in those treated longer duration with MP. There was also a six-fold higher mortality reported in those patients receiving longer treatment [
29]. Of note, the results from these studies have not impacted the current guidelines that advocate the use of MP in spinal cord injury, nor did US Food and Drug Administration grant MP an indication for acute spinal cord injury.
Mortality and morbidity rates are high among patients suffering from bacterial meningitis, specifically from pneumococcal infections [
30]. Adjunctive dexamethasone use in bacterial meningitis has been proposed to be beneficial in reducing mortality rates and hearing loss; but the results from a meta-analysis study do not support the advantage of combining dexamethasone with antimicrobial therapy. Dexamethasone administered at 10 mg IV every 6 hours 15 to 20 minutes before initiating antimicrobial therapy for up to 4 days was not associated with a significant reduction in death, severe neurological sequelae, including bilateral severe deafness or hearing loss, when all patients were included in the analysis. Thus, the benefit of adjunctive dexamethasone for bacterial meningitis remains unproven [
31].
Parenteral administration of high CSs doses may be warranted in neurologic emergencies. High dose CSs are standard of care for those presenting with critically ill condition, such as relapse from neurologic disease crises [
21,
26]. Intravenous MP is the frequently utilized agent to treat major neurologic disease [
32,
33]. In emergency cases, the usual treatment consists of 500-2,000 mg of MP or dexamethasone 10-200 mg IV daily for 3-5 days [
32]. If adequate response is achieved, the steroids can be tapered rapidly or continued for over 1-3 weeks [
34]. Some patients may require a longer steroid maintenance based on the severity of the disease presentation or in patients with large tumor size remnants, the rate of steroid tapering depends on dosage, and duration of therapy [
34]. Because of the many inherent side effects associated with CSs with longer term use, clinicians should carefully evaluate the risk versus benefit of their use and whether pulse steroid would be a better option [
34,
35]. Due to the mineralocorticoid activity of MP, this agent may not be the preferred agent in patients presenting with hypertension, congestive heart failure, or those in whom volume overload is a concern. In these cases dexamethasone, lacks mineralocorticoid activity, can serve as an alternative therapy in place of MP as a first line agent [
34].
CONCLUSIONS
CSs find a myriad of uses in neurological disorders due to their anti-inflammatory and immunosuppressive properties. Although, animal studies have suggested potential benefit of CSs as neuroprotective agents, clinical trials have yet to show convincing beneficial outcomes in this setting. Because of the negative outcomes seen in these large randomized studies, and the vast unwanted side effects associated with CSs, a careful risk benefit analysis should be exercised in the treatment of certain types of neurologic disorders.
Figure 1.
Structures of commonly prescribed synthetic glucocorticoids, and mineralocorticoid.
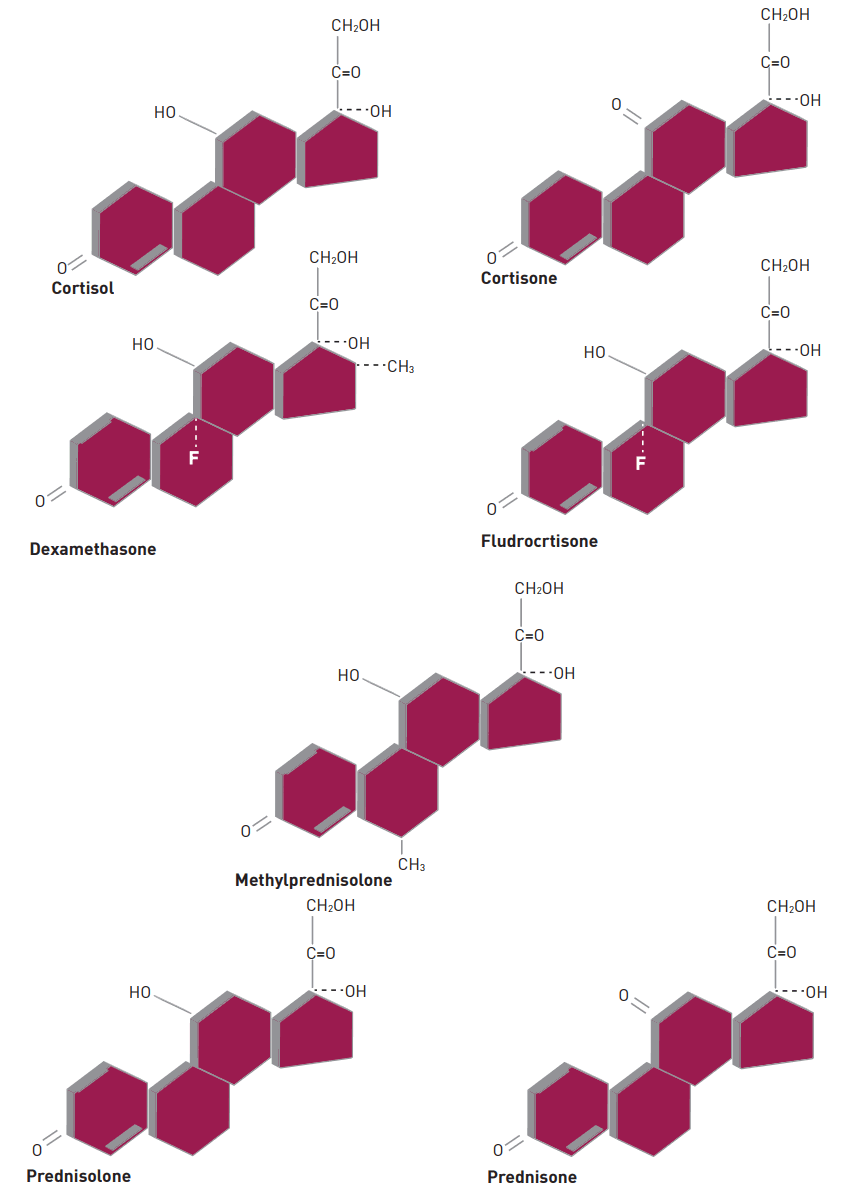
Table 1.
Corticosteroid comparison chart
|
Equivalent glucocorticoid dose (mg) |
Potency relative to hydrocortisone
|
Half-life duration of action (hours) |
|
Anti-inflammatory |
Mineralocorticoid |
|
Glucocorticoids |
|
|
|
|
|
Short acting |
|
|
|
|
|
Hydrocortisone |
20 |
1 |
1 |
8-12 |
|
Cortisone acetate*
|
25 |
0.8 |
0.8 |
8-12 |
|
Intermediate acting |
|
|
|
|
|
Prednisone |
5 |
4 |
0.8 |
12-36 |
|
Prednisolone |
5 |
4 |
0.8 |
12-36 |
|
Methylprednisolone*
|
4 |
5 |
0.5 |
12-36 |
|
Long acting |
|
|
|
|
|
Dexamethasone*
|
0.75 |
30 |
0 |
36-54 |
|
Mineralocorticoid |
|
|
|
|
|
Fludrocortisone |
0 |
15 |
150 |
24-36 |
REFERENCES
1. Schimmer BP, Funder JW. ACTH, Adrenal Steroids, and Pharmacology of the Adrenal Cortex. In: Brunton LL, Chabner BA, Knollmann BC, editor. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill; 2011. p. 1209-35.
2. Hench PS, Kendall EC, Slocumb CH, Polley HF. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone; compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc Staff Meet Mayo Clin 1949;24:181-97.

3. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med 2005;353:1711-23.


4. Vischer TL, Sinniger M, Ott H, Gerster JC. A randomized, double-blind trial comparing a pulse of 1000 with 250 mg methylprednisolone in rheumatoid arthritis. Clin Rheumatol 1986;5:325-6.


5. Garber EK, Fan PT, Bluestone R. Realistic guidelines of corticosteroid therapy in rheumatic disease. Semin Arthritis Rheum 1981;11:231-56.

6. Laan RF, van Riel PL, van de Putte LB, van Erning LJ, van’t Hof MA, Lemmens JA. Low-dose prednisone induces rapid reversible axial bone loss in patients with rheumatoid arthritis. A randomized, controlled study. Ann Intern Med 1993;119:963-8.


7. Migeon CJ, Lawrence B, Bertrand J, Holman GH. In vivo distribution of some 17-Hydroxycorticosterioids between the plasma and red blood cells of man. J Clin Endocrinol Metab 1959;19:1411-9.

8. Pugeat MM, Dunn JF, Nisula BC. Transport of steroid hormones: interaction of 70 drugs with testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab 1981;53:69-75.


9. Ballard PL. Delivery and transport of glucocorticoids to target cells. Monogr Endocrinol 1979;12:25-48.


10. Diederich S, Eigendorff E, Burkhardt P, Quinkler M, Bumke- Vogt C, Rochel M, et al. 11beta-hydroxysteroid dehydrogenase types 1 and 2: an important pharmacokinetic determinant for the activity of synthetic mineralo- and glucocorticoids. J Clin Endocrinol Metab 2002;87:5695-701.


11. Koetz KR, van Rossum EFC, Ventz M, Diederich S, Quinkler M. BclI polymorphism of the glucocorticoid receptor gene is associated with increased bone resorption in patients on glucocorticoid replacement therapy. Clin Endocrinol (Oxf) 2013;78:831-7.


12. Giordano R, Marzotti S, Berardelli R, Karamouzis I, Brozzetti A, D’Angelo V, et al. BClI polymorphism of the glucocorticoid receptor gene is associated with increased obesity, impaired glucose metabolism and dyslipidaemia in patients with Addison’s disease. Clin Endocrinol (Oxf) 2012;77:863-70.


13. Meikle AW, Weed JA, Tyler FH. Kinetics and interconversion of prednisolone and prednisone studied with new radioimmunoassays. J Clin Endocrinol Metab 1975;41:717-21.


14. Meikle AW, Tyler FH. Potency and duration of action of glucocorticoids. Effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. Am J Med 1977;63:200-7.


15. Liddle GW. Studies of structure-function relationships of steroids. II. The 6 alpha-methylcorticosteroids. Metabolism 1958;7(4 Pt 2):405-15.

16. Dulin WE. Anti-inflammatory activity of delta1-9alpha-fluorohydrocortisone acetate. Proc Soc Exp Biol Med 1955;90:115-7.


17. Soffer LJ, Orr RH. Symposium: newer hydrocortisone analogs. Metabolism 1958;7(4 Pt 2):383-6.

18. Dulin WE, Barnes LE, Glenn EM, Lyster SC, Collins EJ. Biologic activities of some C21 steroids and some 6 alpha-methyl C21 steroids. Metabolism 1958;7(4 Pt 2):398-404.

19. Fauci AS. Mechanisms of the Immunosuppressive and antiinflammatory effects of glucorcorticosteroids. J Immunopharmacol 1978-1979;1:1-25.

21. Yokoyama H, Takabatake T, Takaeda M, Wada T, Naito T, Ikeda K, et al. Up-regulated MHC-class II expression and γ-IFN and soluble IL-2R in lupus nephritis. Kidney Int 1992;42:755-63.


22. Kehlet H, Binder C, Blichert-Toft M. Glucocorticoid maintenance therapy following adrenalectomy: assessment of dosage and preparation. Clin Endocrinol (Oxf) 1976;5:37-41.


23. Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet 2004;364:1321-8.


24. Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 2017;80:6-15.


25. Olby N. Current concepts in the management of acute spinal cord injury. J Vet Intern Med 1999;13:399-407.


26. Buttgereit F, Wehling M, Burmester GR. A new hypothesis of modular glucocorticoid actions: Steroid treatment of rheumatic diseases revisited. Arthritis Rheum 1998;41:761-7.


27. Bracken MB, Collins WF, Freeman DF, Shepard MJ, Wagner FW, Silten RM, et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA 1984;251:45-52.


28. Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinalcord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med 1990;322:1405-11.


29. Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 1997;277:1597-604.


30. de Gans J, van de Beek D; European Dexamethasone in Adulthood Bacterial Meningitis Study Investigators. Dexamethasone in adults with bacterial meningitis. N Engl J Med 2002;347:1549-56.


35. Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol 2001;33:289-94.






